Saponification-free process for extracting lithium
A non-saponifiable and extraction technology, applied in the direction of improving process efficiency, can solve problems such as destroying the extractant, and achieve the effects of reducing production cost, reducing acid consumption and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
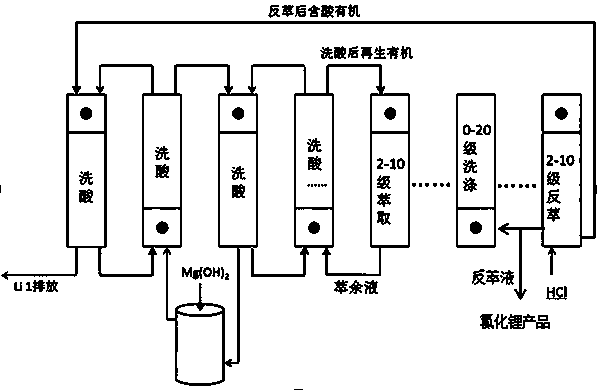
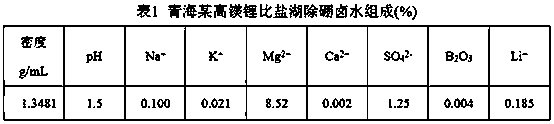
[0026] Add 65 mL of LTBP and 35 mL of kerosene into a 250 mL separating funnel, mix well in the 250 mL separating funnel, add 100 mL of iron-on-iron brine for one extraction (iron on the organic phase, Fe: Li=1.5), let stand to separate the phases, Add 100 mL of brine without iron and boron removal to the organic phase for extraction, a total of 4 extractions (industrial production uses multi-stage countercurrent extraction). Take 6 M HCl and add it to the organic phase for stripping (industrial production uses multi-stage countercurrent stripping), and strip 3 times, 5 mL each time. The organic phase after stripping was washed once with 50 mL of workshop raffinate, and the washing solution was neutralized to pH=2 with magnesium hydroxide, and then washed once. Take 100 mL workshop boron extraction raffinate (without iron brine) for secondary extraction, and determine Li in the raffinate + Concentration, calculate the second extraction rate of 67.2%.
[0027] Under the same ...
Embodiment 2
[0029] Add 15 mL of N503, 35 mL of LTBP and 50 mL of kerosene into a 250 mL separating funnel, mix well in the 250 mL separating funnel, add 100 mL of iron brine for one extraction (iron on the organic phase, Fe: Li=1.3), let stand Separate the phases, add 100mL brine to the organic phase that has not been treated with iron and boron for extraction, and extract a total of 3 times (industrial production uses multi-stage countercurrent extraction). Use workshop stripping solution (Li + 38.5g / L,H +0.551 g / L) and washed 3 times, 3 mL each time (industrial production uses multi-stage countercurrent washing). Take 7 M HCl and add it to the organic phase for back extraction, back extraction 3 times, 5 mL each time (industrial production uses multi-stage countercurrent back extraction); the organic phase after back extraction is washed twice with workshop raffinate, 25 mL each time , after the second washing, neutralize it with magnesium oxide to pH = 1, then introduce it into the s...
Embodiment 3
[0031] Add 40 mLDIBK, 10 mLTBP and 50 mL kerosene into a 250 mL separating funnel, mix well in the 250 mL separating funnel, add 100 mL iron brine for one extraction (iron on the organic phase, Fe: Li=1.3), let stand Separate the phases, add 100mL brine to the organic phase that has not been treated with iron and boron for extraction, and extract a total of 3 times (industrial production uses multi-stage countercurrent extraction). Take 5 M HCl and add it to the organic phase for back extraction, back extraction 3 times, 5 mL each time (industrial production uses multi-stage countercurrent back extraction); the organic phase after back extraction is washed once with workshop raffinate, 20 mL, The washed solution is neutralized with slaked lime to pH = 1, and then introduced into the system for the second washing. After the second washing, the solution is neutralized with slaked lime to pH = 1, and then introduced into the system for the third wash (Industrial production uses m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com