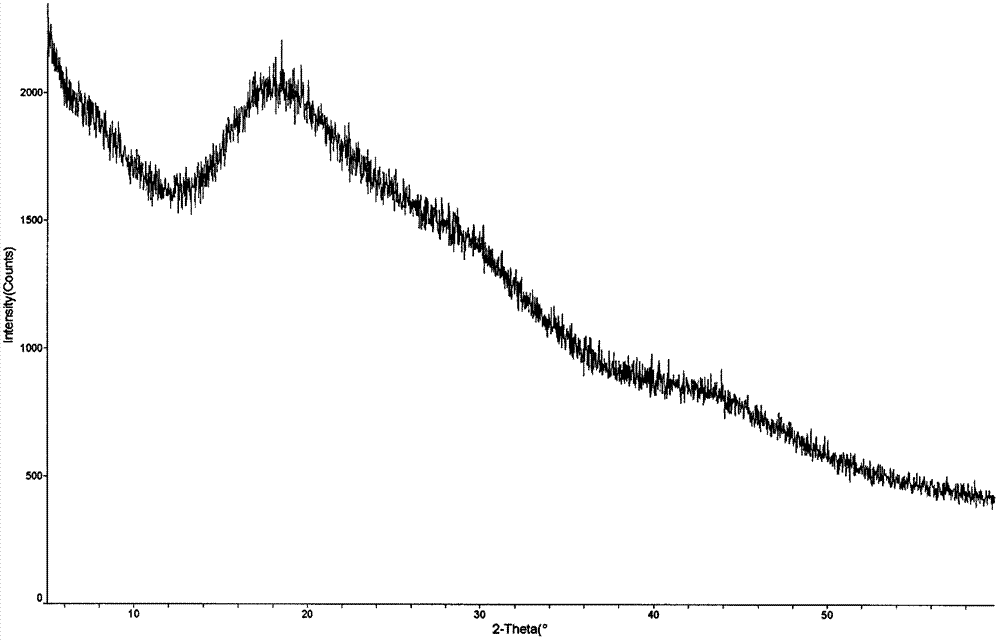
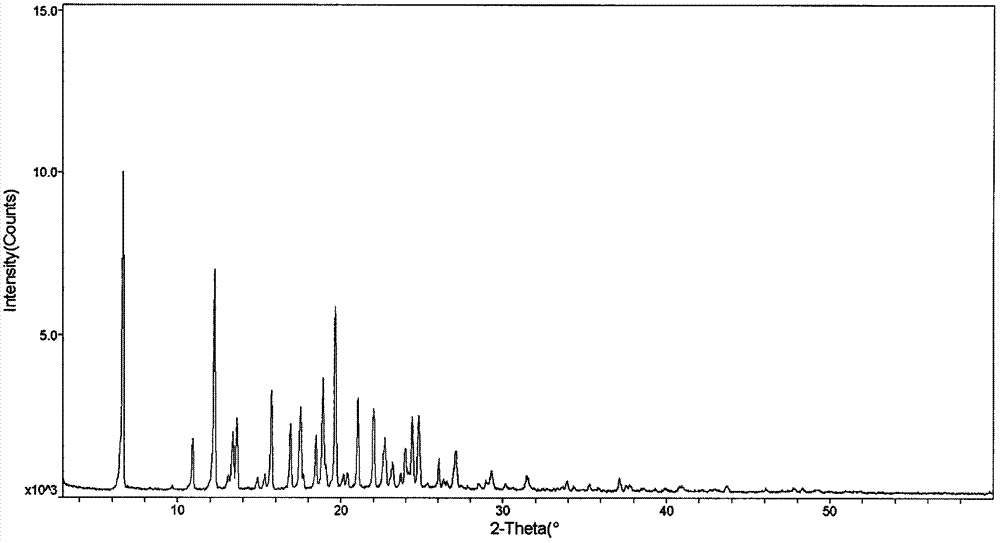
Crystal, pharmaceutical composition and application of compound N-butyl-2,2'-imino-bis(8-quinolinamine)
A technology of imino and quinoline amines, applied in the field of crystals of double-type derivatives, can solve the problems of no crystallography and research, and achieve the effects of easy packaging, good water solubility and fat solubility, and excellent physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] An example of the N-butyl-2,2'-imino-bis(8-quinolinamine) crystal of the present invention, the N-butyl-2,2'-imino of this embodiment -The preparation method of bis(8-quinolinamine) crystals is:
[0052] (1) Take 8.0g of crude N-butyl-2,2'-imino-bis(8-quinolinamine), add dichloromethane to dissolve it, and add 400mL of 1mol / L HCl to the solution, Precipitating N-butyl-2,2'-imino-bis(8-quinolinamine);
[0053] (2) Filtration, add 100 mL of sodium bicarbonate aqueous solution with a concentration of 5% by mass to the obtained filter cake to dissolve it, then extract with ethyl acetate and concentrate the extract to obtain a refined product, which is identified as N by nuclear magnetic resonance analysis -Butyl-2,2'-imino-bis(8-quinolinamine);
[0054] (3) Take 200mg of the above N-butyl-2,2'-imino-bis(8-quinolinamine) refined product, dissolve it in 30mL methanol, place it in an environment of 0-5°C, and let it stand still for solvent Slowly volatilize to obtain a yellow crys...
Embodiment 2
[0063] An example of the N-butyl-2,2'-imino-bis(8-quinolinamine) crystal of the present invention, the N-butyl-2,2'-imino of this embodiment -The preparation method of bis(8-quinolinamine) crystals is:
[0064] (1) Take 10.0g of crude N-butyl-2,2'-imino-bis(8-quinolinamine), add chloroform to dissolve it, and add 600mL of HCl with a concentration of 1mol / L to the solution, Precipitating N-butyl-2,2'-imino-bis(8-quinolinamine);
[0065] (2) Filtration, add 400 mL of sodium carbonate aqueous solution with a concentration of 5% by mass to the obtained filter cake to dissolve it, then extract with ethyl acetate, concentrate the extract to obtain a refined product, which is identified as N-butyl by NMR -2,2'-imino-bis(8-quinolinamine);
[0066] (3) Take 300 mg of the above N-butyl-2,2'-imino-bis(8-quinolinamine) refined product, heat it to dissolve it in 40mL ethanol, and slowly lower the temperature to -5~0℃. Standing at temperature, a yellow crystalline substance precipitated, that i...
Embodiment 3
[0074] An example of the N-butyl-2,2'-imino-bis(8-quinolinamine) crystal of the present invention, the N-butyl-2,2'-imino of this embodiment -The preparation method of bis(8-quinolinamine) crystals is:
[0075] (1) Take 12.0 g of N-butyl-2,2'-imino-bis(8-quinolinamine) crude product, add dichloromethane to dissolve it, and add 1mol / L HCl to the solution until it no longer produces precipitation;
[0076] (2) Filtration, add 300 mL of sodium hydroxide solution with a mass percentage concentration of 1% to the obtained filter cake to dissolve it, then extract with ethyl acetate and concentrate the extract to obtain a refined product, which is identified as N-butyl by NMR Group-2,2'-imino-bis(8-quinolinamine);
[0077] (3) Take 500 mg of the above N-butyl-2,2'-imino-bis(8-quinolinamine) refined product, heat it to dissolve it in 40mL methanol, and slowly decrease the temperature to 0-5℃, at this temperature After standing still, a yellow crystalline substance precipitated, that is, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com