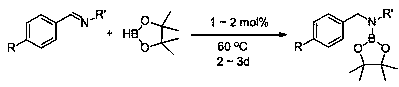Application of Trimethylolene Rare Earth Complex in Catalytic Hydroboration of Imine and Borane
A technology for catalyzing imines and rare earth locenes, which is applied in the directions of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one: La[CpMe] 3 Catalytic synthesis of boronate from benzylidene aniline and pinacol borane
[0029] Under an inert gas atmosphere, add catalytic La[CpMe] to the reaction flask after dehydration and deoxygenation 3 50 mg, add 0.5 mL of tetrahydrofuran, then take 75 μL (0.02 mmol) with a pipette gun and add to another reaction bottle, then add pinacol borane (174 μL, 1.2 mmol) with a pipette gun, and then add benzylidene aniline (0.1812 g, 1 mmol), after reacting at 60°C for 2 days, pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 90%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.33 – 7.17 (m, 10H, ArH), 4.73 (s, 2H, NCH 2 ), 1.33 (s, 12H, CH 3 ).
Embodiment 2
[0030] Embodiment two: Nd[CpMe] 3 Catalytic synthesis of boronate from benzylidene aniline and pinacol borane
[0031] Under an inert gas atmosphere, add catalytic Nd[CpMe] to the reaction flask after dehydration and deoxygenation 3 50 mg, add 0.5 mL of tetrahydrofuran, then pipette 76 μL (0.02 mmol) into another reaction bottle, then add pinacol borane (174 μL, 1.2 mmol) with a pipette, and then add benzylidene Benzene (0.1812 g, 1 mmol), after reacting at 60 °C for 2 days, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 84%. The NMR data of the product are the same as in Example 1.
Embodiment 3
[0032] Embodiment three: Y[CpMe] 3 Catalytic synthesis of boronate from benzylidene aniline and pinacol borane
[0033] Under an inert gas atmosphere, add catalytic Y[CpMe] to the reaction flask after dehydration and deoxygenation 3 50 mg, add 0.5 mL of tetrahydrofuran, then pipette 65 μL (0.02 mmol) into another reaction bottle, then add pinacol borane (174 μL, 1.2 mmol) with a pipette, and then add benzyl Aniline (0.1812 g, 1 mmol), after reacting at 60°C for 3 days, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 82%. The NMR data of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com