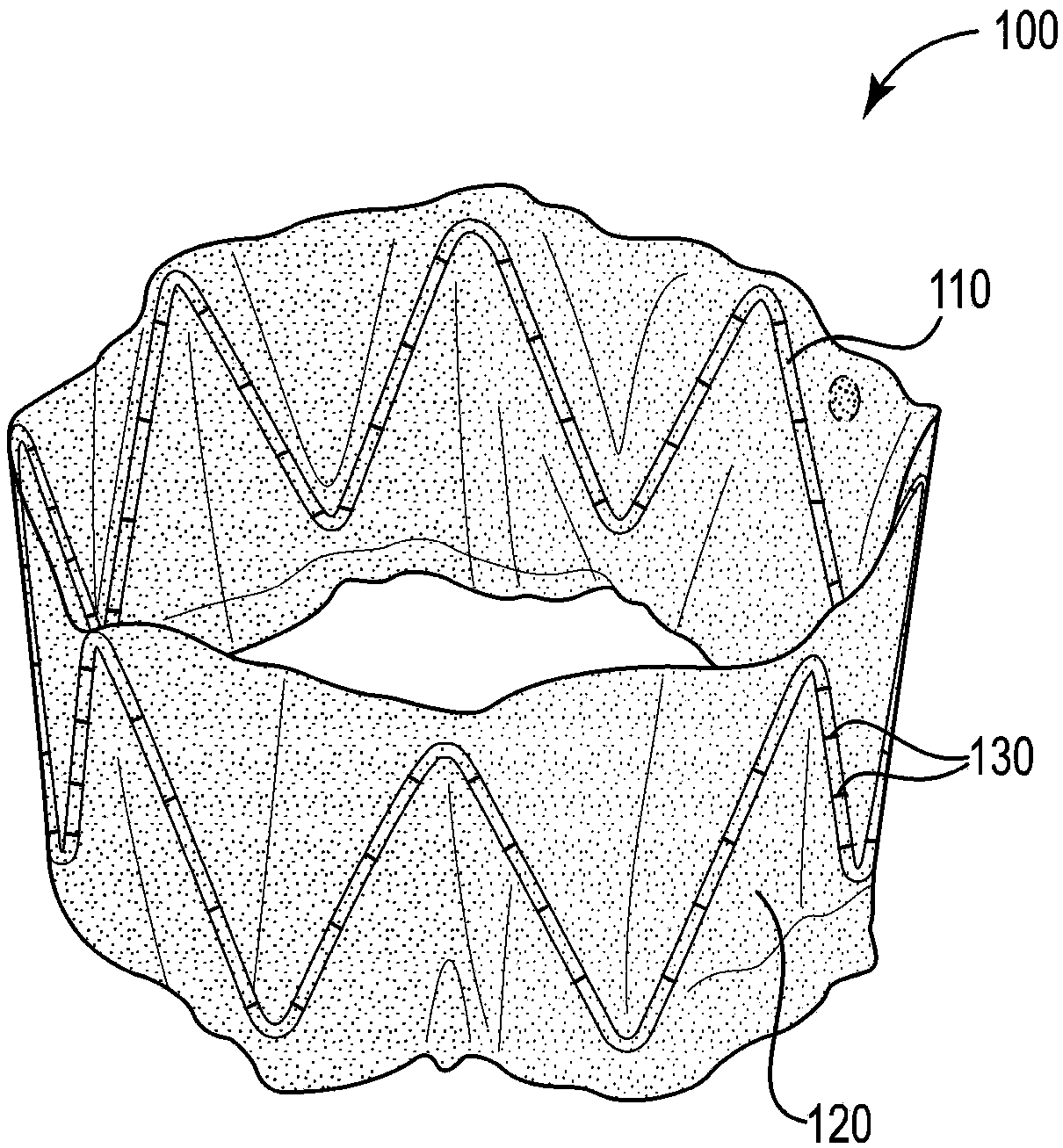
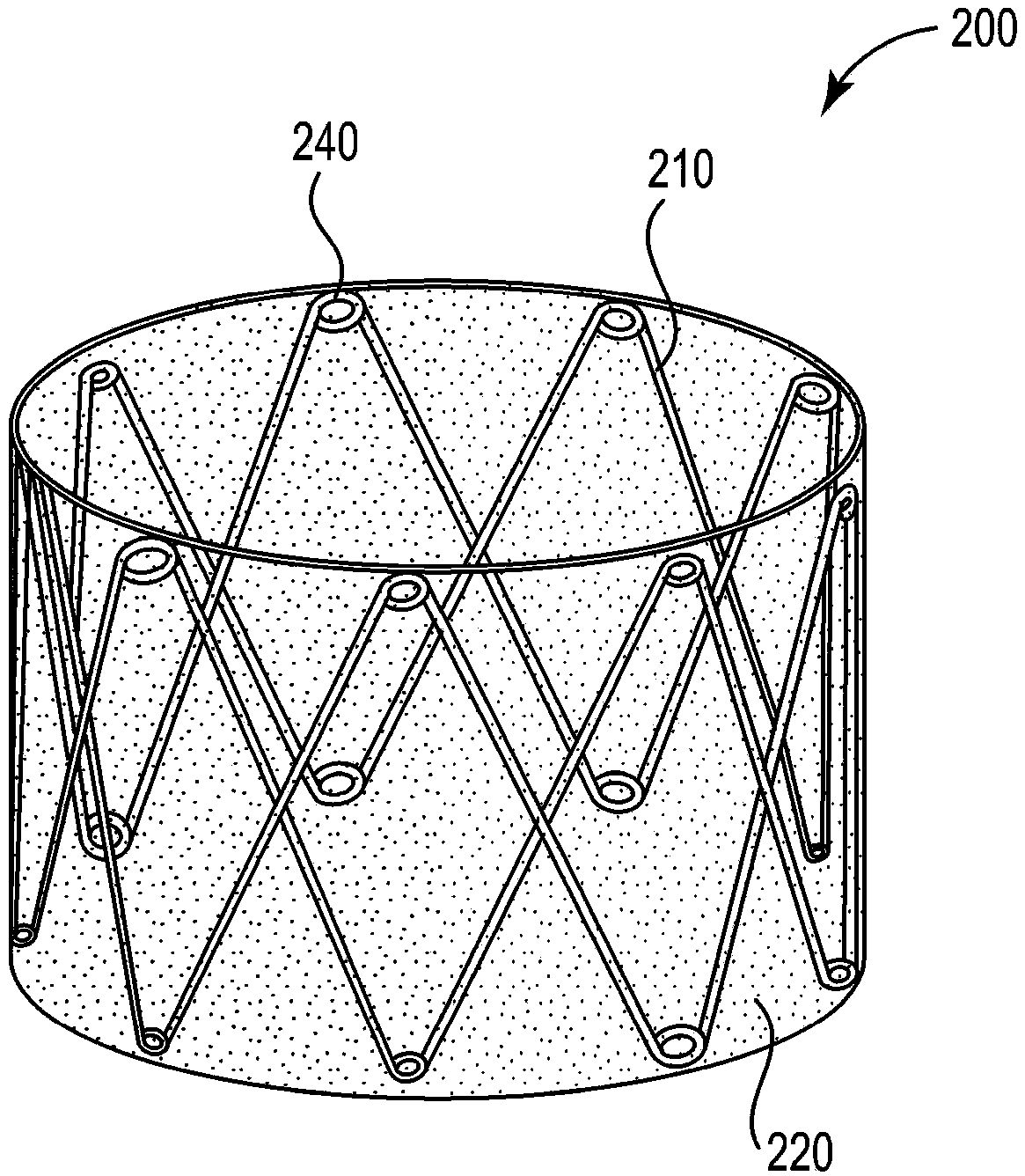
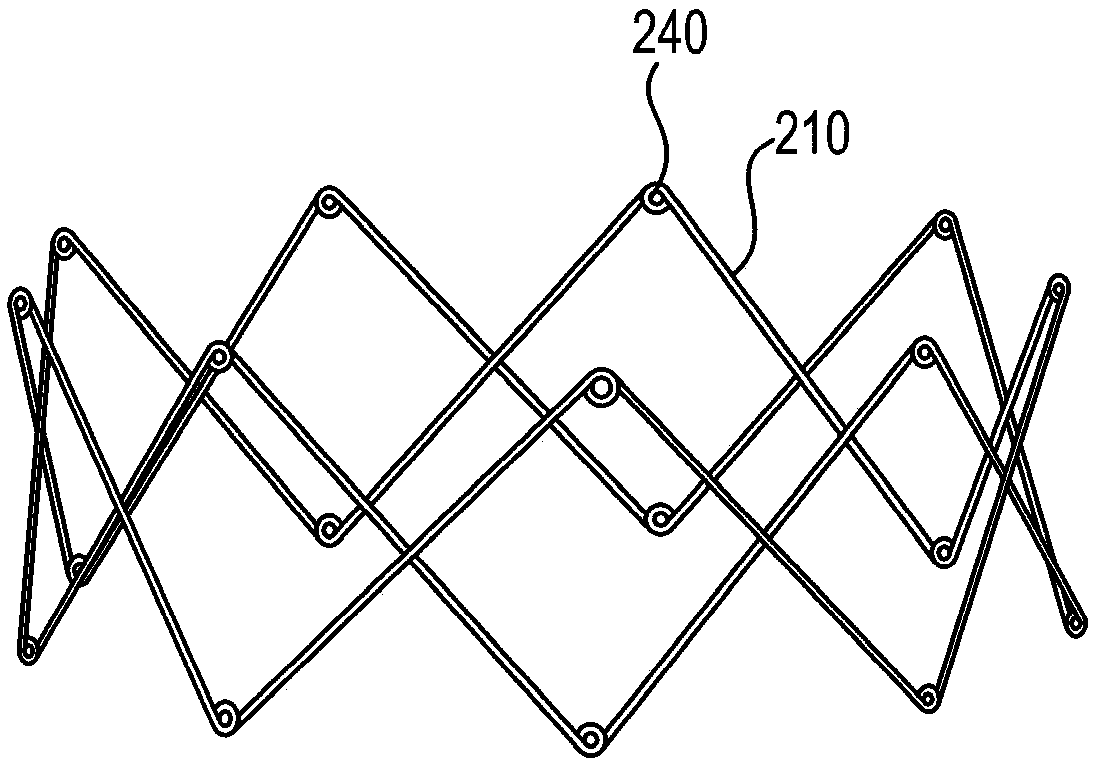
Sinus and nasal stent
A sinus and nasal technology, applied in stents, nose implants, pharmaceutical formulations, etc., can solve the problems of sinus tissue irritation, patient inconvenience and cost, difficulty in providing compression size, expansion size and expansion force, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0078] Base support construction
[0079] Hand-fabricated from a length of 0.38 mm diameter 300 series hard stainless steel medical wire (Malin Co., Cleveland, Ohio) with figure 1 The base bracket 110 is similar to the base bracket. Use UNITEK TM A model 101 welder (3M Unitek, Monrovia, CA) welded the ends of the wires together. The completed base scaffold has a diameter of approximately 3 cm, a strut angle of 32°, a strut length of 2 cm, and 7 peaks and 7 valleys.
example 2
[0081] Polyethylene Sleeve Attachment
[0082] Formed similar to the figure 1 The cannulated stent of the stent 100 in the stent has a layer of LOCTITE on the inner surface of the polyethylene film TM 3972 Light Curing Adhesive (Henkel Corp., Rocky Mount, Conn.). The base scaffold was radially compressed sufficiently to reduce its circumference to approximately 7 cm such that the polyethylene film ends overlapped by approximately 1 cm. Use DYMAX TM BLUEWAVE TM A 200 UV curing spotlight (Dymax Corp., Torrington, Conn.) cured the adhesive under 10 seconds of UV light exposure.
example 3
[0084] coating formulation
[0085] Preparation 1. Using PEVA beads (Sigma-Aldrich Co., St Louis, MO) containing 40% by weight vinyl acetate and having a melt index of 8 g / 10 min at 190 °C / 2.16 kg, (PEVA) was dissolved in 20 ml of tetrahydrofuran (THF) (Sigma-Aldrich Co.) to prepare a 5% (w / v) PEVA solution. An 8 mg portion of triamcinolone acetonide (TA) (Sigma-Aldrich Co.) was added and dissolved in the solution.
[0086] Preparation 2. Using PEVA beads having a molecular weight of 337,000 as measured by gel permeation chromatography (Sigma-Aldrich Co.) and poly(butyl methacrylate) (PBMA) powder, by dissolving 1 g of PEVA and 0.5 g of PBMA in 20 ml of THF A solution containing 5% (w / v) PEVA and 2.5% (w / v) PBMA was prepared. 8 mg of TA was partially added and dissolved in the solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com