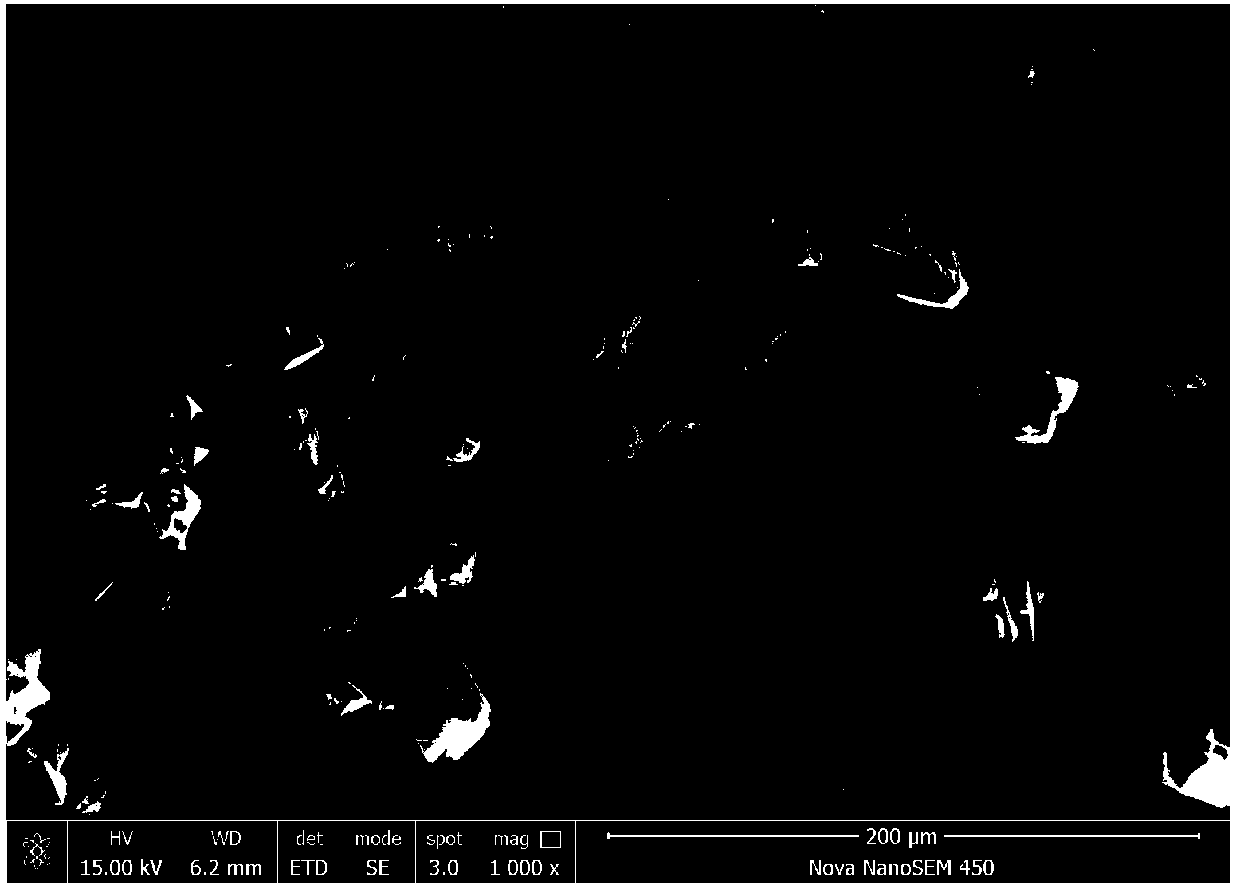
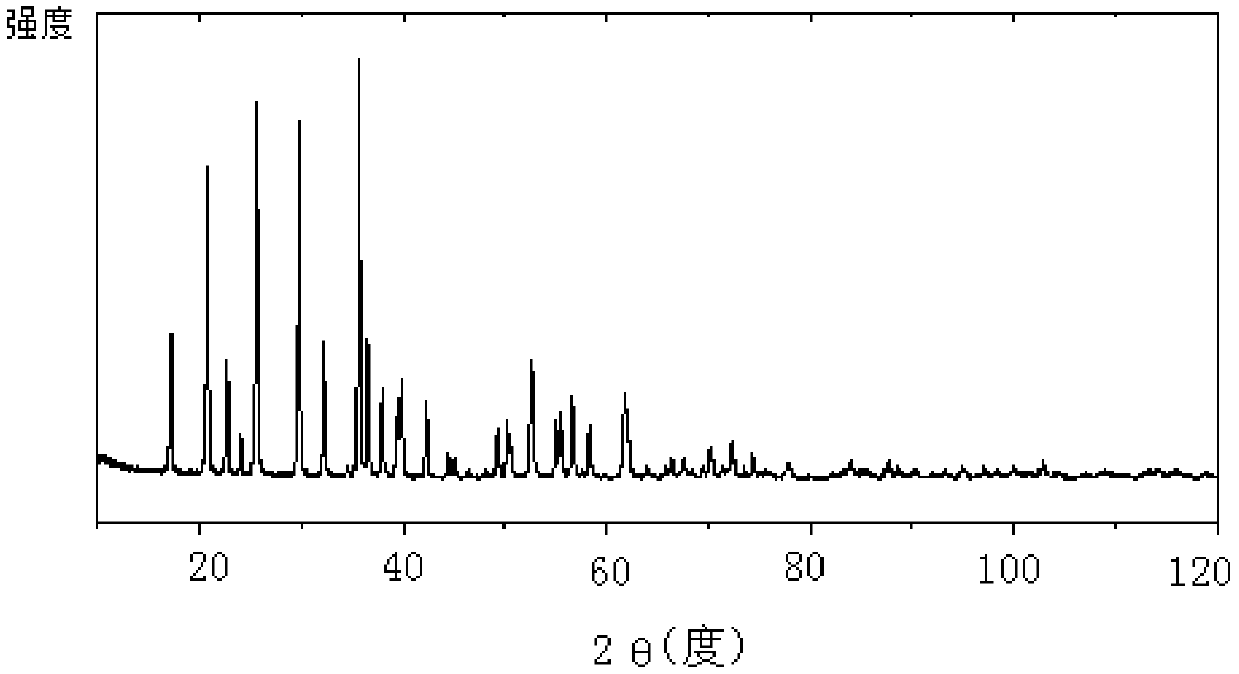
Method for preparing high-density lithium iron phosphate from siderite
A technology of lithium iron phosphate and siderite, which is applied in the field of electrochemistry, can solve the problems of high risk factor, difficult operation, impurity elements, and long process, so as to save energy-consuming steps, improve electrical conductivity, and reduce costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment one is example with the siderite that contains 1mol iron carbonate, the concentration of phosphoric acid is 0.2mol / L, and the concentration of hydrogen peroxide is 10wt%, and concrete preparation method is:
[0036] Step 1: Dissolving Siderite
[0037] According to the molar ratio of iron and pure phosphoric acid is 2:7, directly add H 3 PO is calculated as 3.5 mol of phosphoric acid. At this time, the reaction temperature is 40°C, and the reaction time is 5 hours. After fully reacting, an iron solution is obtained;
[0038] The second step: the iron solution is mixed with P(DMDAAC-AM)
[0039] Add the iron solution obtained in the first step into the P(DMDAAC-AM) solution and stir well to make the two mix evenly. The mass ratio of siderite to P(DMDAAC-AM) is required to be 10:1;
[0040] The third step: the iron solution reacts with hydrogen peroxide
[0041] According to the molar ratio of iron to pure hydrogen peroxide is 2:1, add H 2 o 2 Calculated a...
Embodiment 2
[0046] Embodiment two is example with 1mol siderite (in iron carbonate), the concentration of phosphoric acid is 0.3mol / L, and the concentration of hydrogen peroxide is 20wt%, and concrete preparation method is:
[0047] Step 1: Dissolving siderite.
[0048] According to the molar ratio of iron to pure phosphoric acid is 2:4, directly add H 3 PO is calculated as 2 mol of phosphoric acid, and the concentration of phosphoric acid is 0.3 mol / L. At this time, the reaction temperature is 70°C, and the reaction time is 3 hours. After fully reacting, an iron solution is obtained;
[0049] The second step: the iron solution is mixed with P(DMDAAC-AM)
[0050] Add the iron solution obtained in the first step into the P(DMDAAC-AM) solution and stir well to make the two evenly mixed. The mass ratio of siderite to P(DMDAAC-AM) is required to be 20:1.
[0051] The third step: the iron solution reacts with hydrogen peroxide.
[0052] According to the molar ratio of iron to pure hydrogen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com