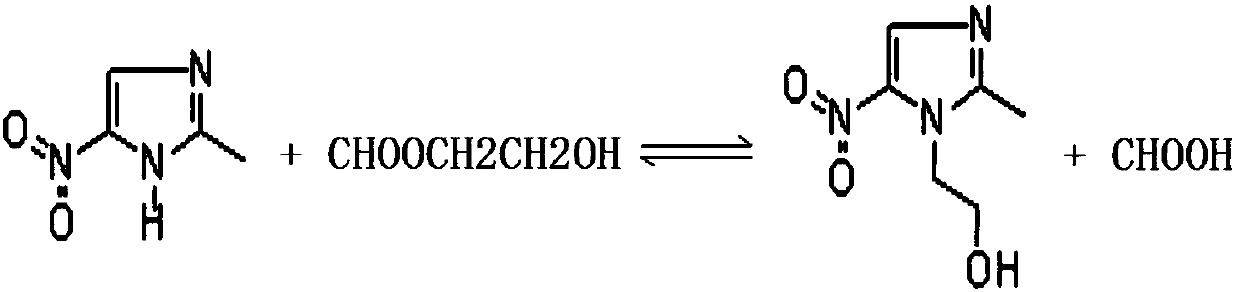Method for applying formic acid glycol ester in metronidazole production, device for achieving method and method for applying device
A technology of glycol ester and formate, applied in the direction of organic chemistry, etc., can solve the problem that the utilization rate of ethylene oxide does not exceed 25%, and achieve the effect of reducing the amount of formic acid and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
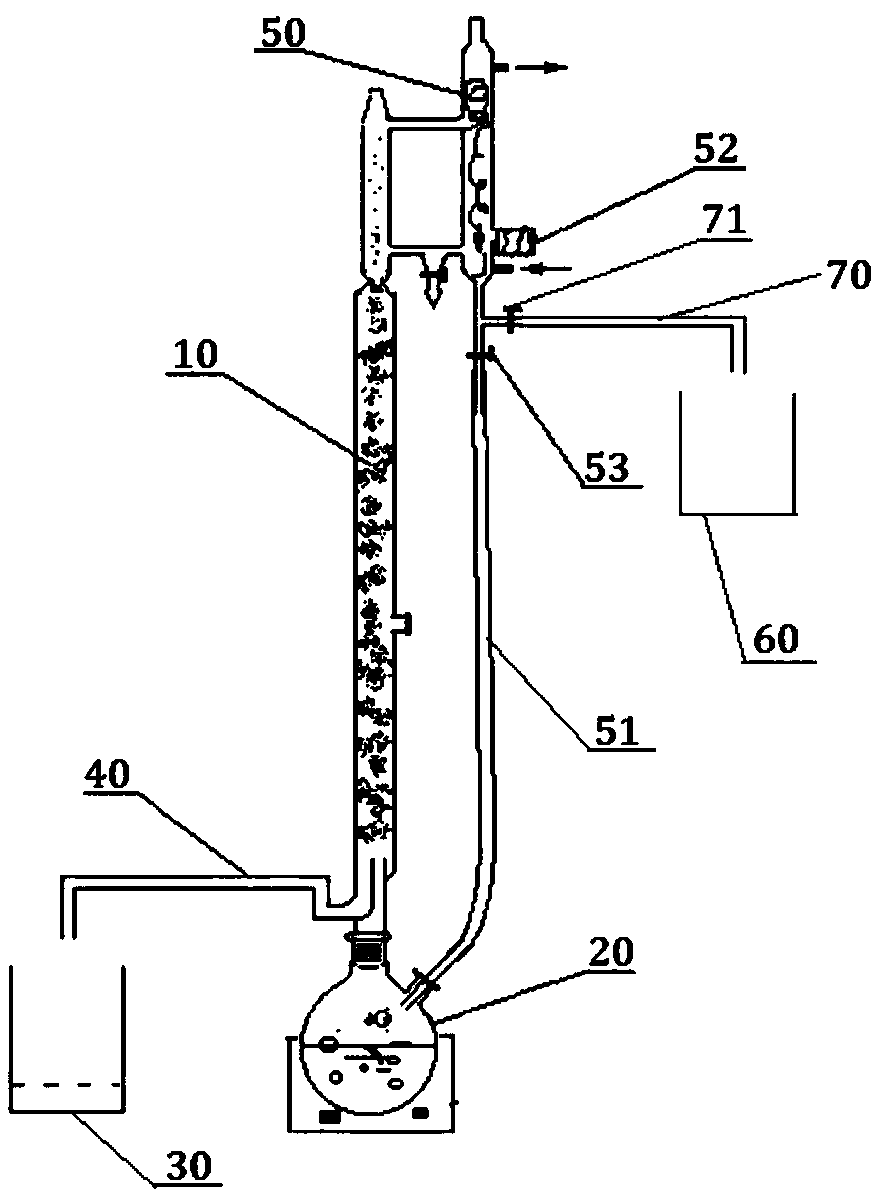
[0062] Step 1): Add 2-methyl-5-nitroimidazole into the reaction kettle 20, add dropwise formic acid solvent with a concentration of 85% to dissolve 2-methyl-5-nitroimidazole, and add cyclic Oxyethane, add sulfuric acid with a concentration of 98% at the same time, raise the temperature to 85-95°C, and react for 1h to obtain a hydroxylated solution;
[0063] Step 2): close the reflux valve 51, open the outlet valve 71, add methanol, control the temperature within a range below 10°C higher than the boiling point of methyl formate, carry out esterification reaction, distill, and condense the steam that evaporates to obtain methyl formate , and collected by the light component liquid collection bottle 60;
[0064] Step 3): The temperature rises to a range below 10°C above the boiling point of methanol, distills methanol, and collects it in the heavy fraction liquid collection bottle 30;
[0065] Step 4): After the hydroxylation solution is cooled to 10°C, adjust the pH to 10 with...
Embodiment 2
[0073] Step 1): Add 2-methyl-5-nitroimidazole into the reaction kettle 20, add dropwise formic acid solvent with a concentration of 85% to dissolve 2-methyl-5-nitroimidazole, and add cyclic Oxyethane, add sulfuric acid with a concentration of 98% at the same time, raise the temperature to 85-95°C, and react for 1h to obtain a hydroxylated solution;
[0074] Step 2): close the reflux valve 51, open the outlet valve 71, add methanol, control the temperature within a range below 10°C higher than the boiling point of methyl formate, carry out esterification reaction, distill, and condense the steam that evaporates to obtain methyl formate , and collected by the light component liquid collection bottle 60;
[0075] Step 3): The temperature rises to a range below 10°C above the boiling point of methanol, distills methanol, and collects it in the heavy fraction liquid collection bottle 30;
[0076] Step 4): After the hydroxylation solution is cooled to 10°C, adjust the pH to 10 with...
Embodiment 3
[0084] Step 1): Add 2-methyl-5-nitroimidazole into the reaction kettle 20, add dropwise formic acid solvent with a concentration of 85% to dissolve 2-methyl-5-nitroimidazole, and add cyclic Oxyethane, add sulfuric acid with a concentration of 98% at the same time, raise the temperature to 85-95°C, and react for 1h to obtain a hydroxylated solution;
[0085] Step 2): close the reflux valve 51, open the outlet valve 71, add methanol, control the temperature within a range below 10°C higher than the boiling point of methyl formate, carry out esterification reaction, distill, and condense the steam that evaporates to obtain methyl formate , and collected by the light component liquid collection bottle 60;
[0086] Step 3): The temperature rises to a range below 10°C above the boiling point of methanol, distills methanol, and collects it in the heavy fraction liquid collection bottle 30;
[0087] Step 4): After the hydroxylation solution is cooled to 10°C, adjust the pH to 10 with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com