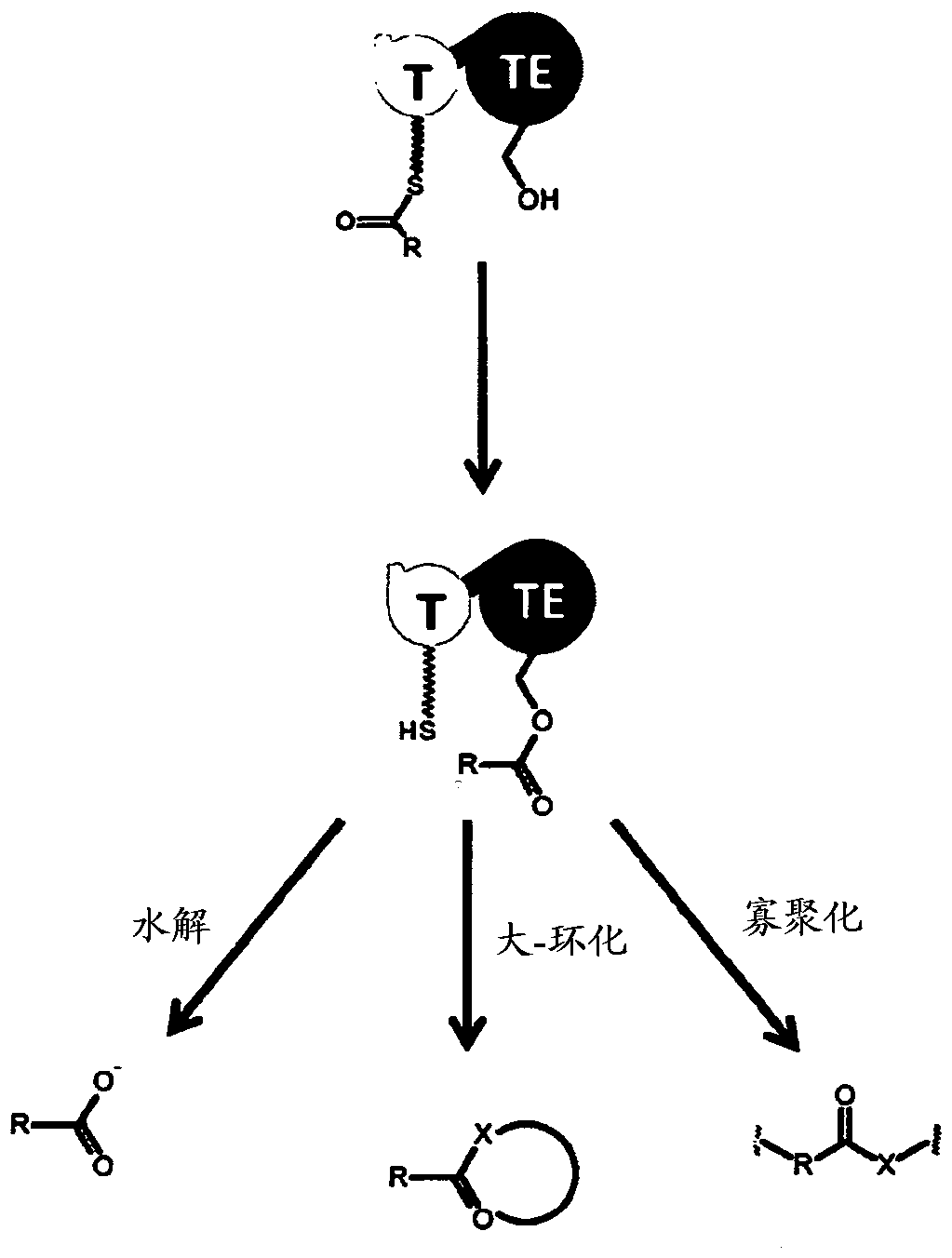
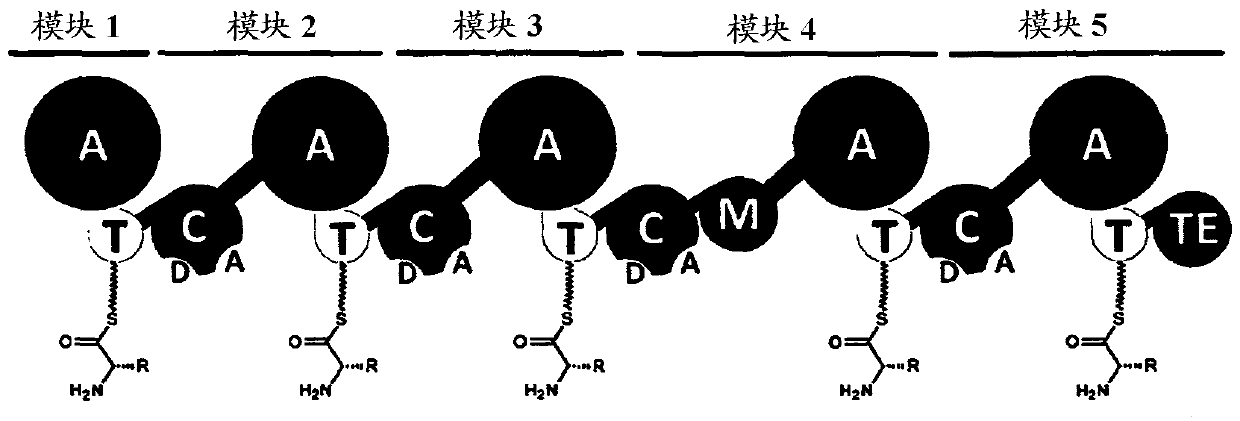
Artificial non-ribosomal peptide synthetases
A technology of variants and structural domains, applied in the direction of enzymes, ligases, drug combinations, etc., can solve problems such as module and/or structural domain compatibility uncertainty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
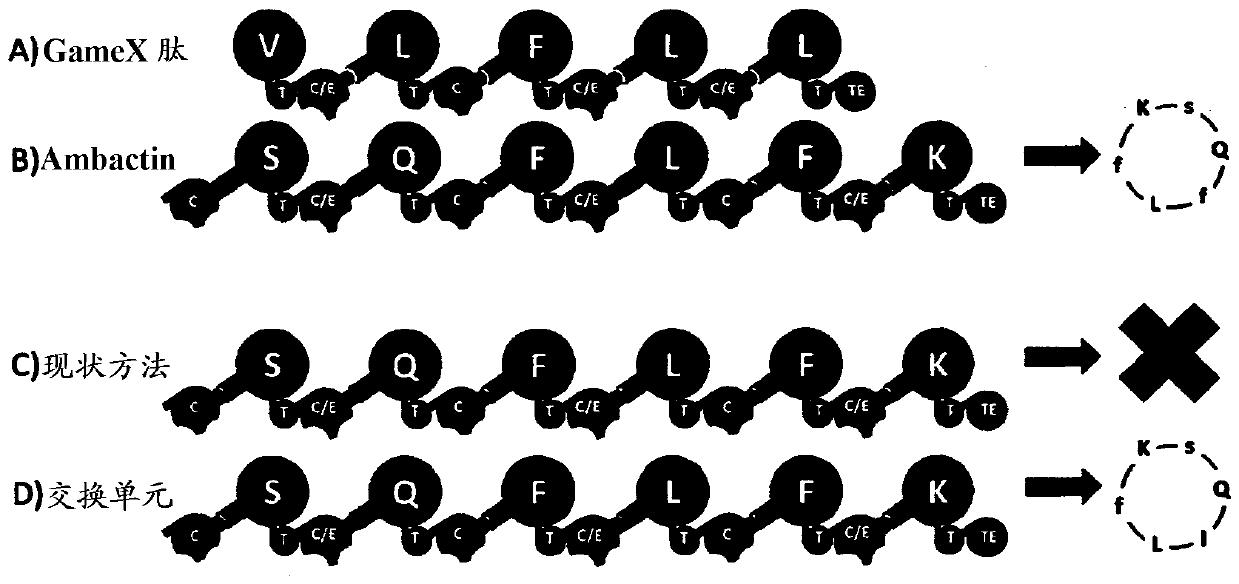
[0066] Example 1: Comparing the present invention (EU concept) with German Patent Application No. 1999151196
[0067] In order to compare the present invention with state-of-the-art methods, the following experiments were performed. In this experiment, we attempted to exchange several domains (yellow) in the Ambactin-producing NRPS AmbS to generate new Ambactin derivatives ( Figure 5 ): on the one hand according to the EU conception ( Figure 5 D), on the other hand according to the prior art method suggested by Marahiel et al. (WO200130985) ( Figure 5 C). Only the present invention yields the desired cyclic peptide. Another recombinant NRPS ( Figure 5 C) shows no generation of any new derivatives.
Embodiment 2
[0068] Example 2: De novo assembly of the NRPS biosynthetic cluster Plu3263 (GxpS) responsible for GameX peptide synthesis
[0069] In support of the accuracy of the present invention, we reassembled the NRPS to generate the GameX peptide from known NRPS building blocks. As predicted, the artificial NRPS was able to produce the desired peptide ( Image 6 ).
Embodiment 3
[0070] Example 3. De novo assembly of the NRPS biosynthetic cluster XtpS responsible for Xenotetrapeptide synthesis and generation of threonine-containing derivatives
[0071] In order to support the accuracy of the present invention and its application in the construction of new artificial NRPS to generate new peptides (from the newly constructed XtpS Figure 7 , artificial NRPS Figure 8 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com