Method for synthesizing D-proline
A synthesis method and a proline technology are applied in the synthesis field of D-proline, can solve problems such as difficult synthesis, high price of D-proline, unfavorable industrial production, etc., achieve high optical purity, low catalyst amount, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
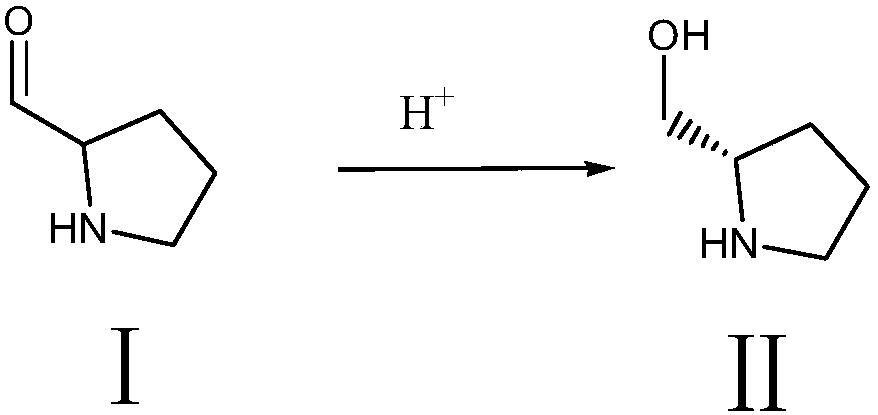
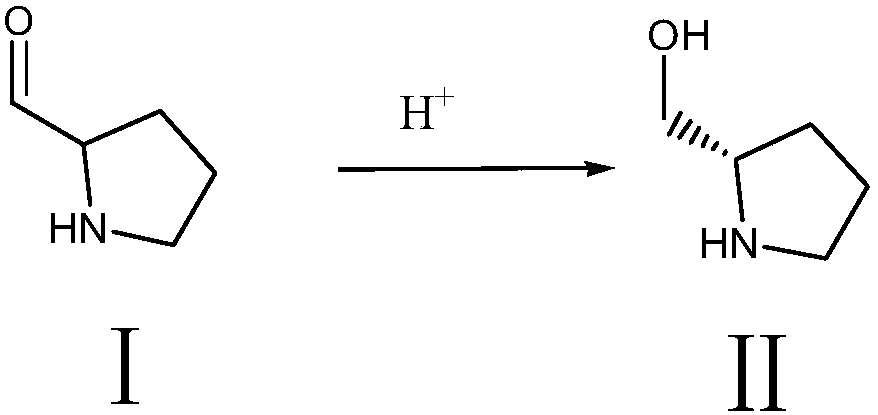
[0027] Step (1) synthesis of intermediate Ⅱ
[0028]
[0029] Add 50g of raw material pyrrolidine-2-carbaldehyde, 6.2g of potassium tert-butoxide and 250ml of absolute ethanol into the hydrogenation kettle, stir evenly, then add 0.075g of (R)-SpiroPAP-Me-Ir, seal it; nitrogen replacement 3 times , replaced by hydrogen for 5 times, pressurized to 2-4MPa, fully stirred, and reacted at 20-25°C for 4 hours; hydrogen should be replenished in time to maintain the pressure, and monitored by HPLC to the point where there is no raw material; after taking out the feed liquid, filter it first to obtain a solid as a catalyst It can be used repeatedly; add 1.5g of activated carbon to the filtrate, keep it warm and stir for 30 minutes. After filtering, the filtrate was evaporated to dryness under reduced pressure at 60°C, and taken twice to dryness with a small amount of ethanol to obtain 50.5 g of oily intermediate II, which was set aside.
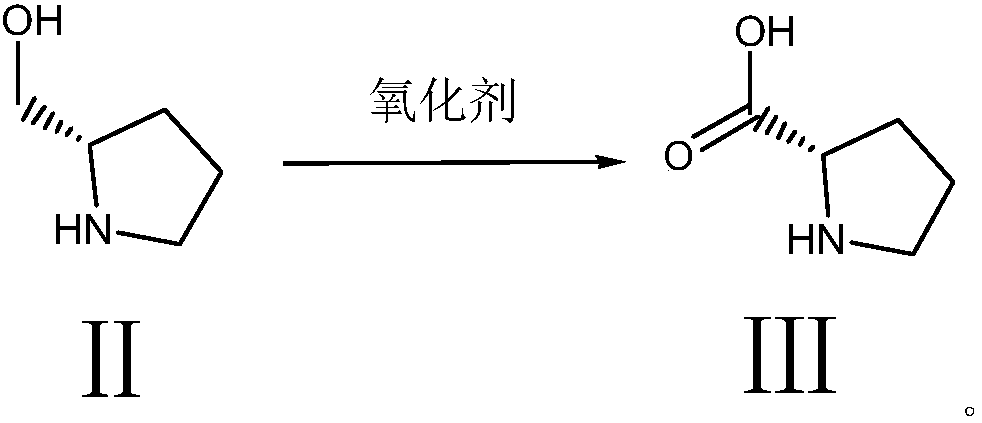
[0030] Step (2) synthetic D-proline
[0031...
Embodiment 2
[0034] In the D-proline synthesis method, the addition ratio of each raw material is an important factor affecting the yield. Keep other conditions of Example 1 constant, only change the addition ratio of each raw material to prepare D-proline, and the yield situation is as follows:
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com