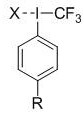
A kind of aryl periodic iodine trifluoromethylation reagent and its preparation and application
A technology of fluorophenyl trifluoromethyl iodine chloride and potassium fluoride, which is applied in the preparation of carbon-based compounds, the preparation of organic compounds, the preparation of hydrogenated polysulfides/polysulfides, etc., can solve the problem of aryl trifluoromethane Due to the lack of direct and effective methods and few types of base periodiodides, the effects of efficient preparation, diverse structures and stable reagents can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
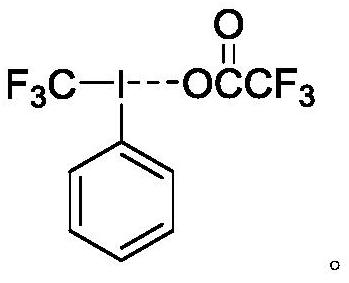
[0048] Preparation of phenyltrifluoromethyl iodine chloride:
[0049] In a 15 mL round bottom flask, add 0.5 mmol bis(trifluoroacetic acid) iodobenzene, under anhydrous nitrogen protection environment, add 0.75 mmol potassium fluoride and 1.0 mL MeCN, add 0.75 mmol trifluoromethyl Trimethylsilane. After stirring for 21 hours, the reaction mixture was poured into a mixed solution of 20 mL of 15% aqueous sodium chloride and 10 mL of acetone at 0 °C. The mixed solution was extracted with dichloromethane (20 mL, 3 times), and the organic phases in the extracted mixed solution were combined and dried over anhydrous magnesium sulfate. After distilling off the solvent, the crude product was washed with petroleum ether to obtain a white solid, phenyltrifluoromethyl iodine chloride, with a yield of 86%.
[0050] NMR and MS characterization of phenyltrifluoromethyl iodine chloride: 1 H NMR (600 MHz, DMSO-d 6 ) δ 8.31(d, J = 8.4 Hz, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.57 (t, J = 7...
Embodiment 2
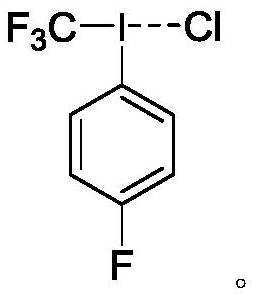
[0052] Preparation of 4-fluorophenyltrifluoromethyl iodine chloride
[0053] In a 15 mL round bottom flask, add 0.5 mmol 4-fluoro-bis(trifluoroacetic acid) iodobenzene, under anhydrous nitrogen protection environment, add 0.75 mmol potassium fluoride and 1.0 mL acetonitrile, add 0.75 mmol Trifluoromethyltrimethylsilane. After stirring for 21 hours, the reaction mixture was poured into a mixed solution of 20 mL of 15% aqueous sodium chloride and 10 mL of acetone at 0 °C. Extract with dichloromethane (20 mL, 3 times). The organic phases in the extracted mixed solution were combined and dried with anhydrous magnesium sulfate. Dry over anhydrous magnesium sulfate. After distilling off the solvent, the crude product was washed with petroleum ether to obtain a white solid, 4-fluorophenyltrifluoromethyl iodine chloride, with a yield of 65%.
[0054] NMR and MS characterization of fluorophenyl trifluoromethyl iodine chloride: 1 H NMR (600 MHz, CDCl 3 , δ): 8.14~8.11 (m, 2H), 7.7...
Embodiment 3
[0056] Preparation of Phenyltrifluoromethyl Iodine Fluoride
[0057] In a 15 mL round bottom flask, add 0.5 mmol bis(trifluoroacetic acid) iodobenzene, under anhydrous nitrogen protection environment, add 0.75 mmol potassium fluoride and 1.0 mL MeCN, add 0.75 mmol trifluoromethyl Trimethylsilane. After stirring for 24 hours, the reaction mixture was poured into a mixed solution of 20 mL of 15% potassium fluoride aqueous solution and 10 mL of acetone at 0 °C. Extract with dichloromethane (20 mL, 3 times). The organic phases in the extracted mixed solution were combined and dried with anhydrous magnesium sulfate. Dry over anhydrous magnesium sulfate. After distilling off the solvent, the crude product was washed with petroleum ether to obtain a white solid, phenyltrifluoromethyl iodine fluoride, with a yield of 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com