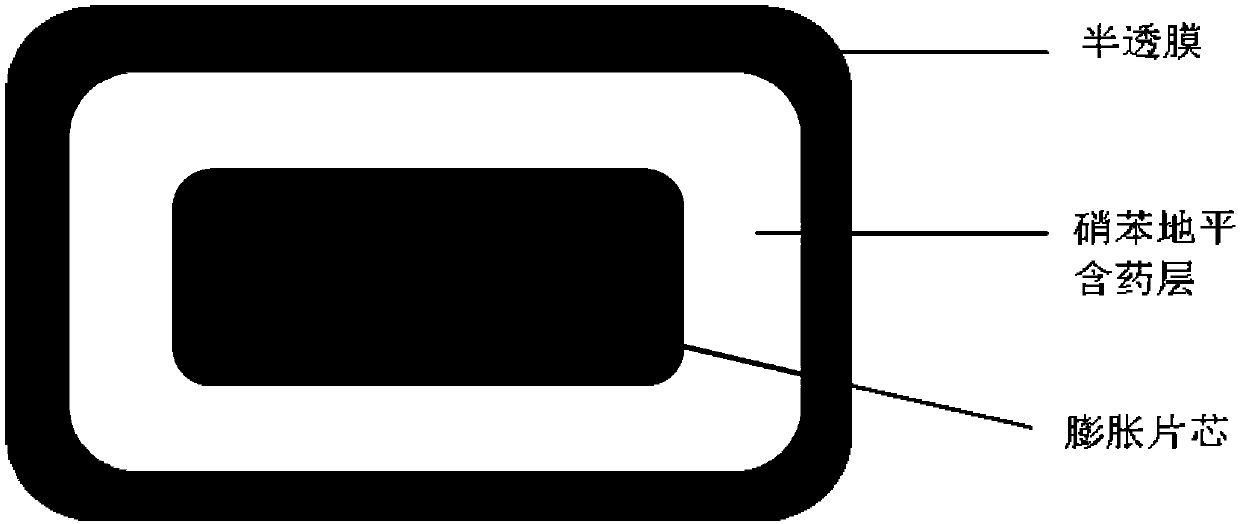
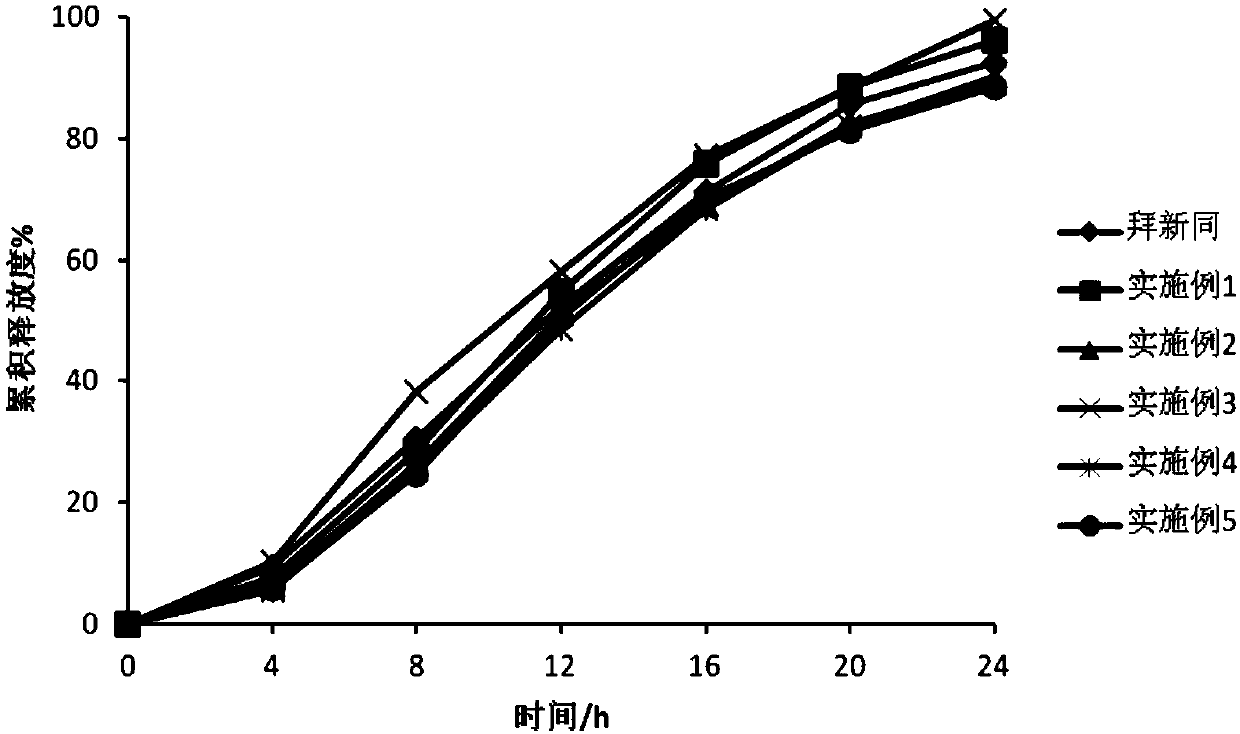
Nifedipine microporous osmotic pump core-encapsulating tablet with expanding tablet core and preparation method thereof
A technology of nifedipine and expansion tablets, which is applied in the field of medicine, can solve the problems such as no reports on the technology of nifedipine microporous osmotic pump tablets, and achieve the goals of avoiding blood drug concentration fluctuations, improving safety, and good internal and external correlations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
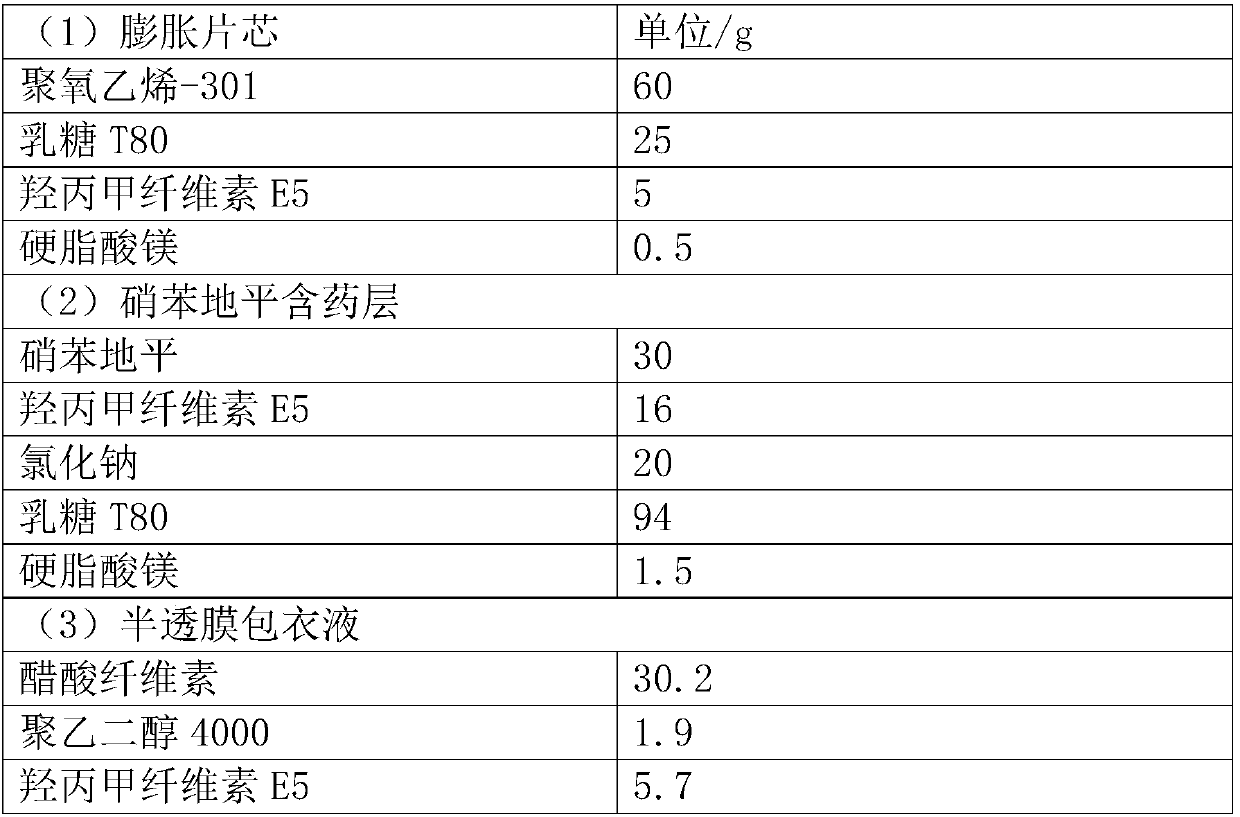
[0030] Prescription (1000 tablets):
[0031]
[0032]
[0033] Preparation process (see figure 1 ):
[0034] 1. Preparation of expanded tablet core:
[0035] Weigh the prescribed amount of polyoxyethylene-301, lactose T80, and hypromellose E5 and mix them in a micro mixer for 10 minutes, add magnesium stearate and mix them for another 2 minutes, and use a ZP-19 rotary tablet press to compress them with a diameter of 6 mm and a hardness of 30-40N shallow concave core.
[0036] 2. Preparation of nifedipine-coated chips
[0037] Avoid light, weigh the prescribed amount of nifedipine, hypromellose E5, sodium chloride, and lactose T80 and mix them in a micro-mixer for 10 minutes, add magnesium stearate and mix them for another 2 minutes, and then use ZPW20 to pack chips and press them. A tablet machine presses the expanded tablet core obtained above and the drug-containing layer of nifedipine to form a shallow concave tablet with a diameter of 8.5mm and a hardness of 60-80N...
Embodiment 2
[0041] Prescription (1000 tablets):
[0042]
[0043]
[0044] Preparation Process:
[0045] 1. Preparation of expanded tablet core:
[0046] Weigh the prescribed amount of polyoxyethylene-coagulant, microcrystalline cellulose PH102, and hypromellose E5 and mix in a micro mixer for 10 minutes, add magnesium stearate and mix for 2 minutes, and use a ZP-19 rotary tablet press to compress the diameter 6mm, shallow concave core with a hardness of 30-40N.
[0047] 2. Preparation of nifedipine-coated chips
[0048] Avoid light operation, weigh the prescribed amount of nifedipine, hypromellose E5, sodium chloride, microcrystalline cellulose PH102, mannitol and mix in a micro mixer for 10 minutes, add magnesium stearate and mix for 2 minutes, Then use a ZPW20 chip-packed tablet press to press the expanded tablet core obtained above and the drug-containing layer of nifedipine to form a shallow concave tablet with a diameter of 8.5mm and a hardness of 60-80N.
[0049] 3. Semi-...
Embodiment 3
[0052] Prescription (10000 tablets):
[0053]
[0054] Preparation Process:
[0055] 1. Preparation of expanded tablet core:
[0056] Weigh the prescribed amount of polyoxyethylene-301, lactose T80, and hydroxypropyl cellulose SSL and mix in a mixer for 15 minutes, add talcum powder and mix for 2 minutes, and use a ZP-19 rotary tablet press to compress the tablet with a diameter of 6mm and a hardness of 30-40N shallow concave core.
[0057] 2. Preparation of nifedipine-coated chips
[0058] Avoid light operation, weigh the prescribed amount of nifedipine, hydroxypropyl cellulose SSL, sodium chloride, lactose T80 and mix in a mixer for 15 minutes, add talcum powder and mix for another 2 minutes, and then use a ZPW20 chip tablet press The expanded tablet core obtained above is laminated with the drug-containing nifedipine to form a shallow concave tablet with a diameter of 8.5mm and a hardness of 60-80N.
[0059] 3. Semi-permeable membrane coating
[0060] Prepare the co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com