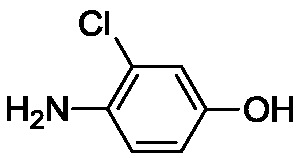
Method for synthesizing 4-amino-3-chlorophenol by means of multiple-temperature-zone continuous flow microchannel reactor
A channel reactor and chlorophenol technology, applied in the field of anticancer drug synthesis in organic synthesis, can solve the problems of difficult preparation, expensive, long reaction route, etc., and achieve the effect of inhibiting hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
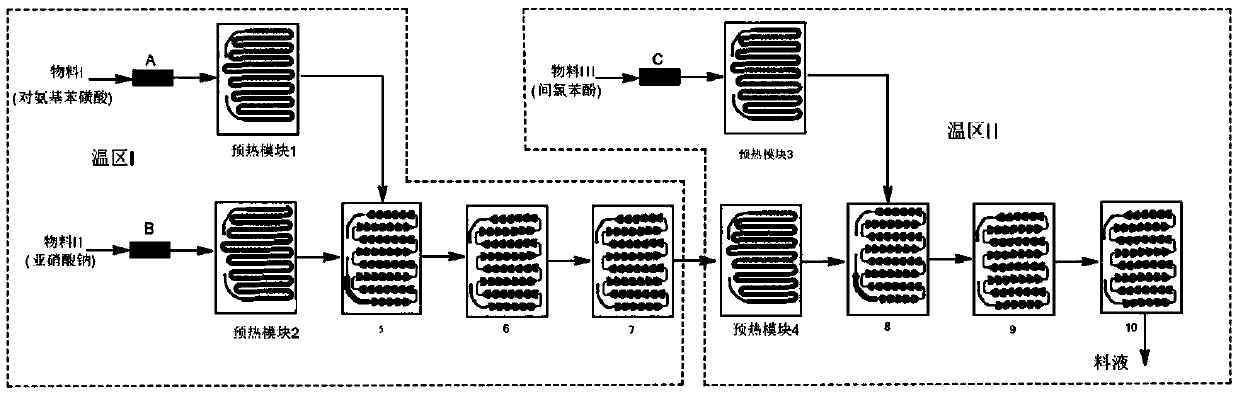
[0039] Embodiment 1. The method described in this embodiment is a kind of multi-temperature zone continuous flow microchannel reactor synthetic 4-amino-3-chlorophenol, comprising the steps:
[0040] (1) Dissolving p-aminobenzenesulfonic acid, sodium nitrite and inorganic alkali I in water, the mixed solution obtained is used as material I, and the concentrated hydrochloric acid dilution is used as material II, and material I and material II carry out diazotization reaction, and the reaction The temperature is -5°C to 20°C, and the residence time is 30s to 60s to generate diazonium salt;
[0041](2) m-chlorophenol and inorganic base II are dissolved in water, and the obtained mixed solution is used as material III, and the diazonium salt obtained in step (1) is coupled with material III, and the reaction temperature is 0° C. to 30° C. The residence time is 20s~45s, and azo compounds are obtained;
[0042] (3) adding a metal reducing agent and a chemical reducing agent to the a...
Embodiment 2
[0045] Embodiment 2. The method for synthesizing 4-amino-3-chlorophenol in multi-temperature zone continuous flow microchannel reactor is described in detail below.
[0046] (1) Take by weighing 173g of p-aminobenzenesulfonic acid and add 2.2L of water, then add 53g of sodium carbonate and 69g of sodium nitrite, stir and dissolve the mixed solution as material I; take by weighing 300g of concentrated hydrochloric acid and add 2L of water, The diluted liquid obtained is as material II; Wherein, the mol ratio of p-aminobenzenesulfonic acid and sodium carbonate is 1:0.5, and the mol ratio of p-aminobenzenesulfonic acid and sodium nitrite is 1:1, and p-aminobenzenesulfonic acid and hydrogen chloride The molar ratio is 1:3.0, in a microchannel reactor, such as figure 1 As shown, material I enters preheating module 1 through liquid flow control pump A, controls metering pump A so that the flow rate of material I is 16mL / min, material II enters preheating module 2 through liquid flow...
Embodiment 3
[0052] Embodiment 3. The method for synthesizing 4-amino-3-chlorophenol in multi-temperature zone continuous flow microchannel reactor.
[0053] (1) Take by weighing 173g of p-aminobenzenesulfonic acid and add 2.2L of water, then add 69g of potassium carbonate and 69g of sodium nitrite, stir and dissolve the mixed solution as material I, take by weighing 300g of concentrated hydrochloric acid and add 2L of water, The diluted liquid obtained is as material II; Wherein, the mol ratio of p-aminobenzenesulfonic acid and salt of wormwood is 1:0.5, and the mol ratio of p-aminobenzenesulfonic acid and sodium nitrite is 1:1, and p-aminobenzenesulfonic acid and hydrogen chloride The molar ratio is 1:3.5, in a microchannel reactor, such as figure 1 As shown, material I enters preheating module 1 through liquid flow control pump A, controls metering pump A so that the flow rate of material I is 20mL / min, material II enters preheating module 2 through liquid flow control pump B, and contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com