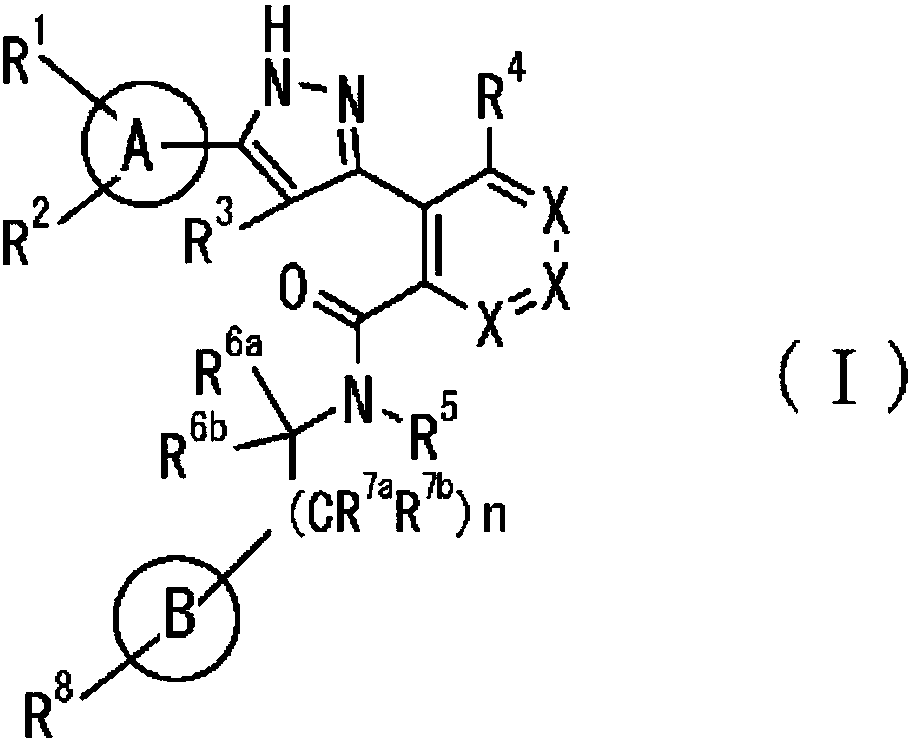
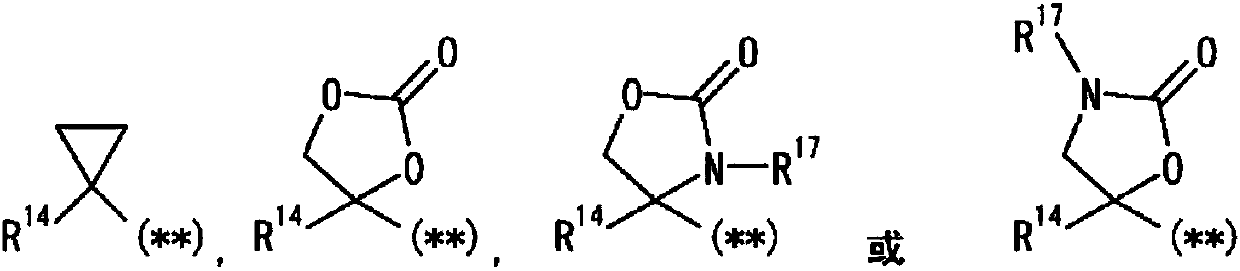
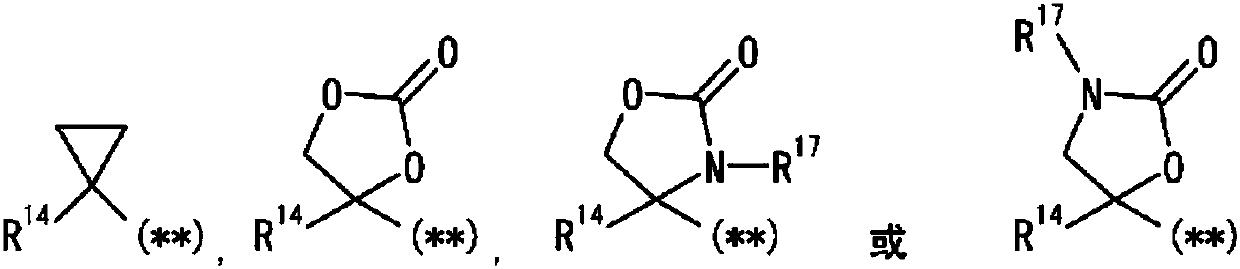
Pyrazole derivative, or pharmaceutically acceptable salt thereof
A pharmacology and compound technology, applied in the field of pyrazole derivatives or their pharmacologically acceptable salts, can solve the problems of different compound structures, no record or suggestion of TRPM8 inhibitors, different structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1-1-1
[0352] 2-[5-(3-Fluorophenyl)-1H-pyrazol-3-yl]benzoic acid
[0353] According to the method described in Journal of Organic Chemistry, 77(8), 3887-3906; 2012 or a method based thereon, the title compound was obtained. The structural formula, spectral data and purification conditions are shown in Table 3.
reference example 1-1-2~1-1-16
[0355] Using the corresponding raw materials, Reference Examples 1-1-2 to 1-1-16 were synthesized by the same method as Reference Example 1-1-1. In addition, the structural formula, spectrum data, and purification conditions are shown in Table 3-Table 4.
reference example 1-2-1
[0357] 4-Ethynyl-1-fluoro-3-methoxybenzene
[0358] To 4-bromo-1-fluoro-3-methoxybenzene (0.513g), trimethylsilylacetylene (0.737g) in tetrahydrofuran (5mL) was added triethylamine (3.795g), bistri Phenylphosphine palladium(II) chloride (0.175g), copper(I) iodide (0.048g), were stirred at 110°C for 1 hour under microwave irradiation. The mixture was passed through a pad of celite. A saturated aqueous ammonium chloride solution was added to the filtrate, and the crude product was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, and the obtained residue was dissolved in tetrahydrofuran (5 mL), and tetra-n-butylammonium fluoride tetrahydrofuran solution (1 mol / L, 5 mL) was added under ice-cooling, followed by stirring for 30 minutes. After adding saturated aqueous ammonium chloride solution to the reaction mixture, the crude product was extracted wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com