A ferrite@graphene hydrogel composite material and its application in the field of electrochemical energy storage
A technology of graphene hydrogel and composite materials, which is applied in the field of new functional materials and electrochemical energy storage, can solve the problems of difficult control of material morphology and performance, undisclosed related performance, complicated operation process, etc., and achieve improved electrochemical performance. Energy storage performance, convenient operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1)CoFe 2 O 4 @Graphene Hydrogel Composite Material Preparation
[0036] 1mmol Co(NO 3 ) 3 , 2mmol Fe(NO 3 ) 3 Mix with 5mmol of sodium acetate, and add 25mL of ethylene glycol to fully stir; then mix with 33mL of graphene oxide with a concentration of 3.5g / L, fully stir and add to the reactor, increase the temperature to 180°C, and keep it for 12 hours. After the reaction, the gel was taken out and washed with a lot of water to obtain CoFe 2 O 4 @Graphene hydrogel composite materials.
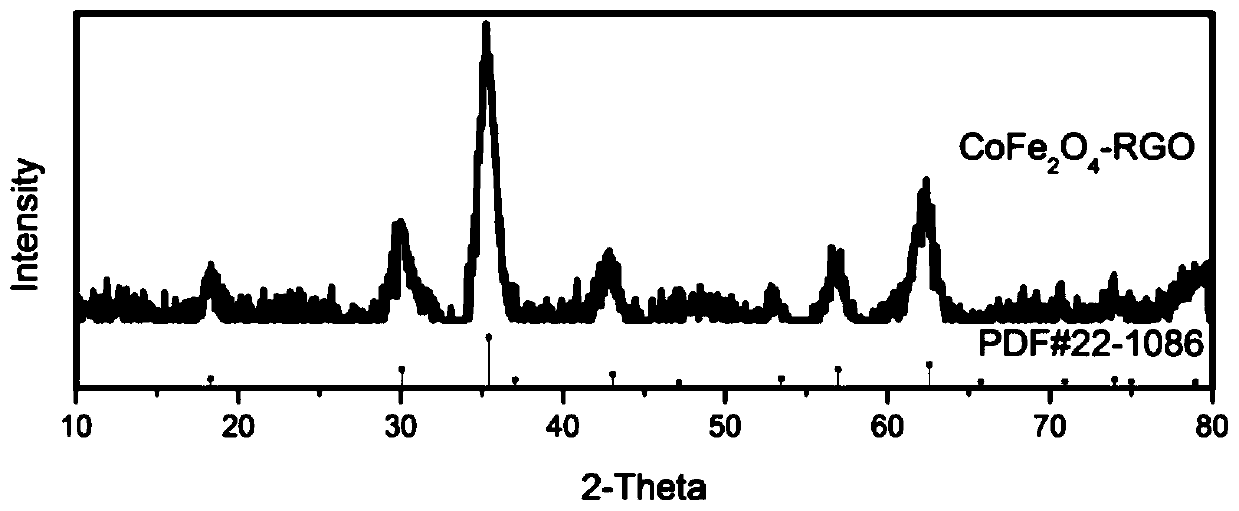
[0037] Attached figure 1 Is the prepared CoFe 2 O 4 @Graphene Hydrogel composite X-ray diffraction pattern. It can be seen from XRD that diffraction peaks appear at 18.3, 30.1, 35.4, 37.1, 43.1, 53.4, 57.0, 62.6 and 74.0° positions, and these diffraction peaks can correspond to CoFe 2 O 4 (111), (220), (311), (222), (400), (331), (422), (511), (400) and (533) crystals in (JCPDS no.22-1086) The above shows that what we have prepared is cobalt ferrite with spinel structure.
[0038] Attached ...
Embodiment 2
[0043] (1)NiFe 2 O 4 @Graphene Hydrogel Composite Material Preparation
[0044] 1mmol Ni(NO 3 ) 3 , 2mmol Fe(NO 3 ) 3 Mix with 4.6mmol of sodium acetate and add 25mL of ethylene glycol to fully stir; then mix with 26mL of 5g / L graphene oxide solution, fully stir and add to the reactor, warm to 220°C, and keep for 10 hours. After the reaction, the gel was taken out and washed with a large amount of water to obtain NiFe 2 O 4 @Graphene hydrogel composite materials.
[0045] Attached Figure 7 Is NiFe 2 O 4 Nano material and NiFe prepared by the invention 2 O 4 @Graphene composite materials at low temperature nitrogen adsorption test results (BET and BJH). From the obtained test results, the NiFe obtained by the present invention can be calculated 2 O 4 @Graphene composite material has a specific surface area of 614.4m 2 / g, than NiFe 2 O 4 Nano materials (179.7m 2 / g.) 3.4 times higher.
[0046] (2)NiFe 2 O 4 @Reduced graphene oxide composite electrode preparation
[0047] The prepar...
example 3
[0048] Example 3: Manganese Ferrite@Graphene Hydrogel Composite
[0049] (1) MnFe 2 O 4 @Graphene Hydrogel Preparation
[0050] 1mol Mn(NO 3 ) 3 , 2mol Fe(NO 3 ) 3 Mix with 4mol sodium acetate, and add 16mL ethylene glycol to fully stir; then mix with 25mL of 4.5g / L graphene oxide solution, fully stir and add into the reactor, warm to 160°C and keep for 18 hours. After the reaction, the gel was taken out and washed with a large amount of water to obtain MnFe 2 O 4 @Graphene hydrogel composite materials.
[0051] Attached Figure 8 MnFe prepared by the invention 2 O 4 @Graphene composite electron transmission image and EDX, you can also see MnFe 2 O 4 Nano particles are more uniformly dispersed and compounded on the graphene sheet.
[0052] (2) MnFe 2 O 4 @Graphene composite electrode preparation
[0053] The prepared MnFe 2 O 4 @Graphene hydrogel composite material, after freeze-drying, is pressed on a foamed nickel electrode of a certain size, used as the electrode material of superc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| mechanical strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com