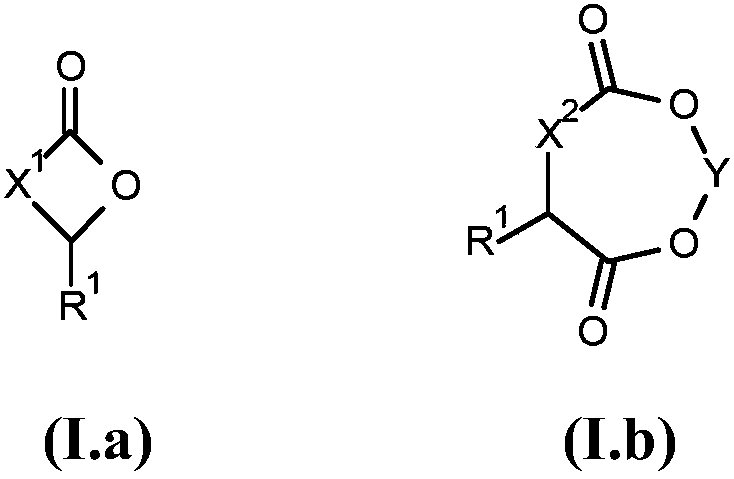
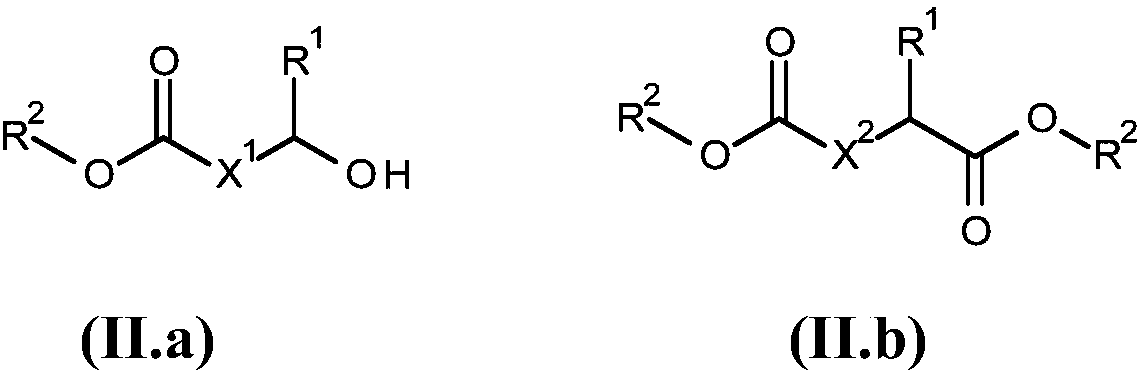
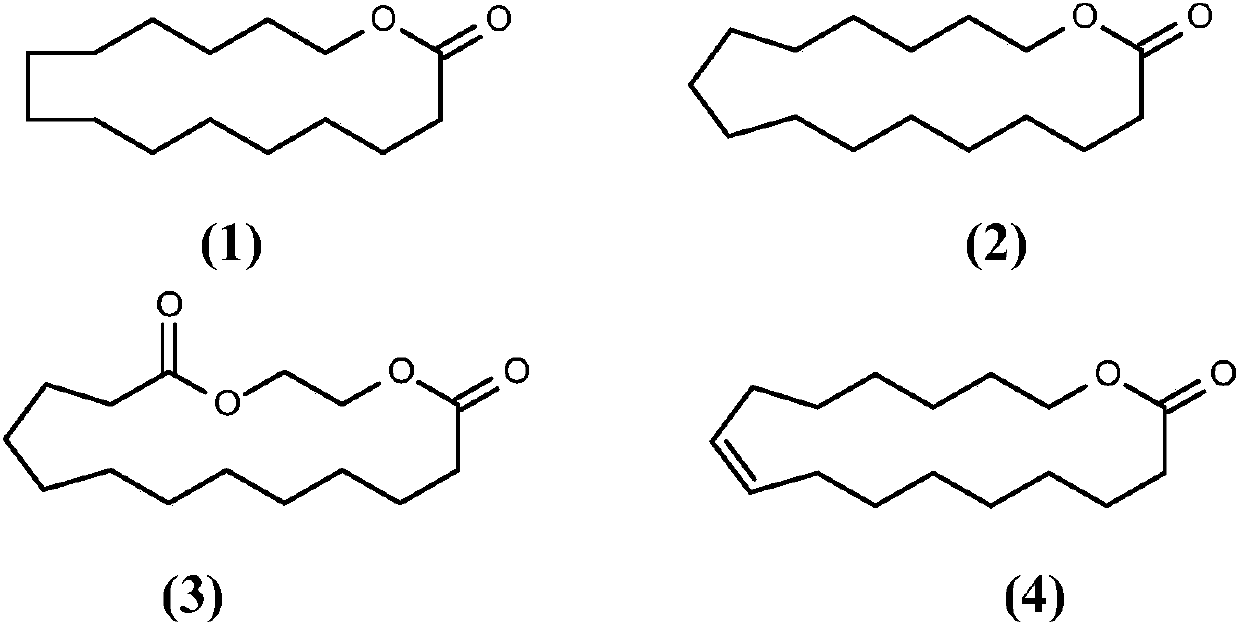
Method for producing cyclic esters
A technology of macrocyclic compounds and general formulas, which is applied in the field of preparation of cyclic esters, and can solve problems affecting catalyst activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0343] Example II.1: Cyclization of butyl 15-hydroxypentadecanoate to 15-cyclopentadecanolide
[0344] At room temperature, 2.48 grams of titanium (IV) isopropoxide (0.01 mol, 0.10 equivalents) were added to 80 g 2000 (PEG 2000). The mixture was heated to 140 to 150°C and the resulting isopropanol was stripped from the solution with nitrogen over about 3 hours. The mixture was then cooled to 120 °C and 36.0 g (0.11 mol) of 15-hydroxypentadecanoic acid butyl ester melting at 70 °C were added, and the mixture was evacuated to 5 mbar and heated to 250 °C over about 20 minutes. ℃. At 250° C., metering of ethylene glycol (approximately 25 ml / h) was started, whereby a mixture of cyclopentadecanolactone and ethylene glycol was distilled off. After about 6 hours, the distillate was monophasic and after phase separation, 25.5 g of cyclopentadecanolide were obtained with a content of 96.8% by weight, which corresponds to a yield of 89.7%. A further 4.3% of product was obtained in t...
Embodiment II
[0345] Example II.2: Cyclization of methyl 15-hydroxypentadecanoate to 15-cyclopentadecanolide
[0346] At room temperature, 2.84 grams of titanium (IV) isopropoxide (0.01 mol, 0.1 equivalents) was added to 80 g E600S. The mixture was heated to 140 to 145°C and the resulting isopropanol was stripped from the solution with nitrogen over about 3 hours. The mixture was then cooled to 120 °C and 28.7 g (0.1 mol) of 15-hydroxypentadecanoic acid methyl ester melting at 70 °C were added and the mixture was evacuated to 5 mbar and heated to 250 °C over about 20 minutes. ℃. It is also optionally cooled to room temperature and the hydroxyester is added at room temperature, and the catalyst can also be stored. At 250° C., metering of ethylene glycol (approximately 28 ml / h) was started, whereby a mixture of cyclopentadecanolactone and ethylene glycol was distilled off. After about 5 hours, the distillate was monophasic and after phase separation, 22.7 g of cyclopentadecanolide were o...
Embodiment III
[0358] Example III.1a: Isolation and preparation of (ω-1)- and ω-hydroxymethyl oleate from fermented sophorolipids
[0359] 160.8 g of sophorolipid aqueous solution was extracted three times with 400 ml of acetate at room temperature. The combined acetate phases were concentrated to yield 68.6 g of residue. The residue was dissolved in 250 g of methanol and 6.9 g of concentrated sulfuric acid were added at room temperature. The batch was then heated at reflux for 10 hours. The reaction solution was cooled and 13.8 g of potassium carbonate was added, and the mixture was after-stirred at room temperature for 30 minutes. The suspension was filtered and the filtrate was concentrated by evaporation. The final weight was 81.6 grams. The product was placed in 400 ml of ethyl acetate and 400 ml of water and extracted. After phase separation, the aqueous phase is extracted with a further 400 ml of acetate. The ethyl acetate phases were combined and concentrated by evaporation to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com