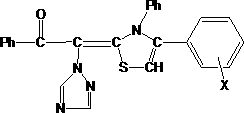
Method for synthesizing thiazole derivative containing acetophenone triazole group in ultrasonic solvent-free manner
An acetophenone triazole group, ultrasonic technology, applied in the direction of organic chemistry, etc., can solve the problems of unrealistic industrial production, difficult to handle, and toxicity of dimethyl sulfoxide, etc., achieve good industrial application prospects, reduce steps, methods simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Accurately weigh homemade α-(1H-1,2,4-triazol-1-yl)acetophenone (synthetic method: take the same molar number of α-bromoacetophenone and 1H-1,2,4 - Triazole and acetone, dropwise add triethylamine of the same number of moles at a certain speed at 0°C. Continue to stir until the white needles no longer continue to precipitate in large quantities after the dropwise addition is completed. Filter off the triethylamine bromate solid, and the filtrate Concentrate to obtain a yellow viscous substance, then dissolve it with chloroform, wash it in a separatory funnel, separate the organic phase, evaporate the chloroform to obtain a light yellow solid, and then recrystallize it with ethanol to obtain a white flaky crystal, the yield is 63.5%.) 1.87 g (0.01mol), 1.37g (0.01mol, slightly excess) phenyl isothiocyanate and 0.68g potassium hydroxide, mix thoroughly in a mortar, transfer to a round bottom flask, fix it in an ultrasonic oscillator Oscillate for 0.5h. Insert an ultrason...
Embodiment 2
[0031]Accurately weigh 1.87 g (0.01 mol) of α-(1H-1,2,4-triazol-1-yl) acetophenone, 1.35 g (0.01 mol) phenyl isothiocyanate and 0.68 g potassium hydroxide, Mix thoroughly in a mortar and transfer to a round-bottomed flask, and fix it in an ultrasonic oscillator to vibrate for 0.5h. Insert an ultrasonic dry-mist nozzle with an atomization particle size of 1-10 µm into the mouth of the round-bottom flask and connect it to the atomizer, and spray α-bromo-p-chloroacetophenone in the form of liquid mist regularly. Set the frequency to 20kHZ~25kHZ, and turn on the ultrasonic response under the rated power of 400w. After the shaking was started, a nebulized α-bromo-p-chloroacetophenone was sprayed into the solids in the flask. When the mist is thick, stop for a period of time and continue to spray α-bromo-p-chloroacetophenone to observe the color change of the reactant solid. When the color of the solid reactant turns yellow completely, and the color does not change, stop spraying ...
Embodiment 3
[0037] Accurately weigh 1.87 g (0.01 mol) of α-(1H-1,2,4-triazol-1-yl) acetophenone, 1.35 g (0.01 mol) phenyl isothiocyanate and 0.68 g (0.012 mol) Potassium hydroxide, mixed thoroughly in a mortar, then transferred to a round bottom flask, fixed in an ultrasonic oscillator and oscillated for 0.5h. Insert an ultrasonic dry-mist nozzle with an atomization particle size of 1-10 μm into the mouth of the round-bottom flask and connect it to the atomizer, and regularly spray α-bromo-2,5-dichloroacetophenone in the form of liquid mist. Set the frequency to 20kHZ~25kHZ, and turn on the ultrasonic response under the rated power of 400w. After the shaking was started, a mist of α-bromo-2,5-dichloroacetophenone was sprayed into the solids in the flask. When the mist is thick, stop for a period of time and continue to spray α-bromo-2,5-dichloroacetophenone, and observe the color change of the reactant solid. When the color of the solid reactant has completely changed to yellow and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com