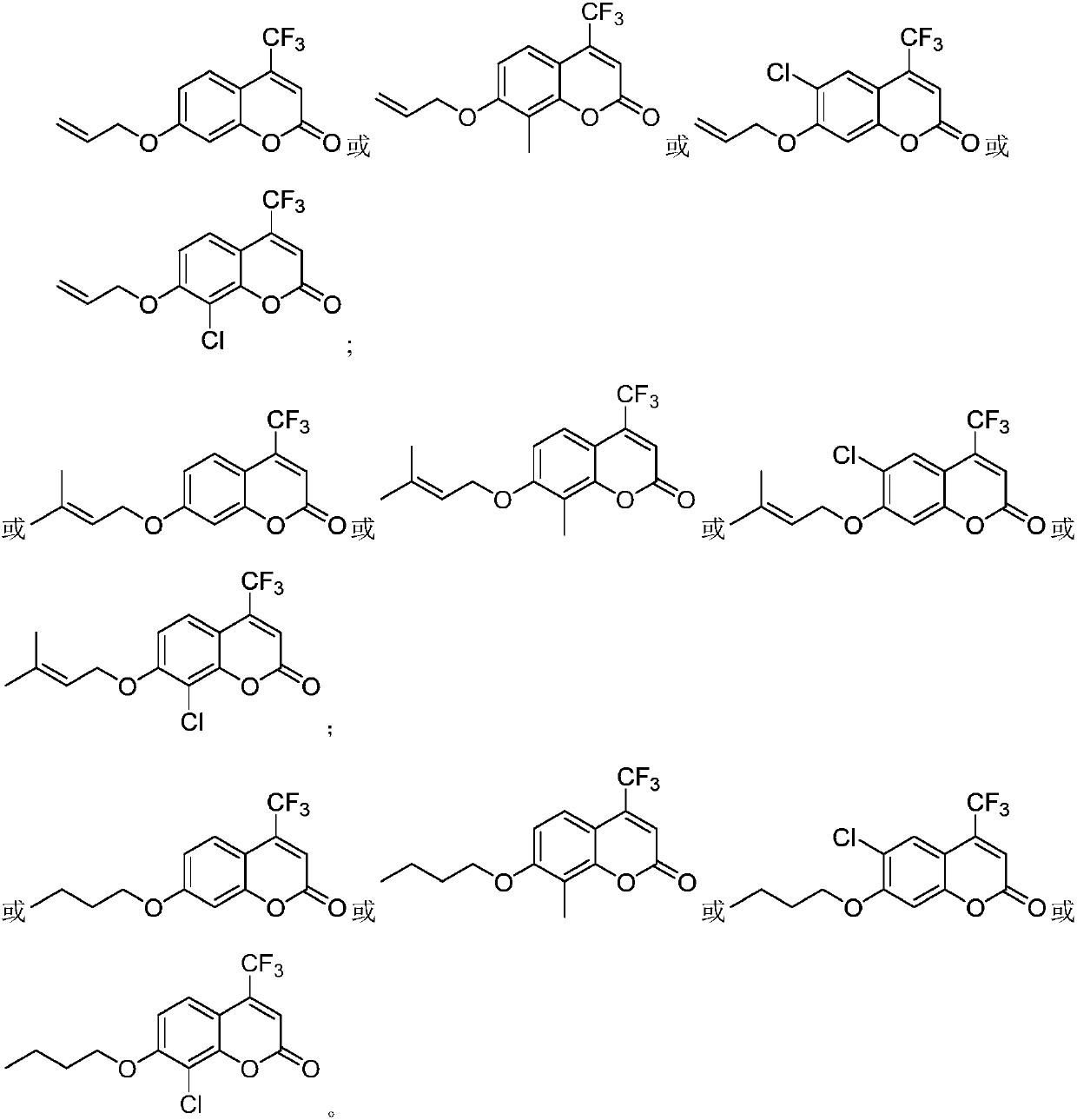
4-trifluoromethyl-7-hydroxycoumarin derivative as well as preparation method and application thereof
A technology of hydroxycoumarin and trifluoromethyl, applied in the field of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of 4-trifluoromethyl-7-hydroxycoumarin (a-1)
[0056] 1. Put 30mL of concentrated sulfuric acid in a three-necked flask and cool to 0°C in an ice-salt bath.
[0057] 2. 11.0 g of resorcinol and 12 mL of ethyl trifluoroacetoacetate were uniformly mixed under ultrasonic to obtain a mixed solution of the two.
[0058] 3. Drop the mixed solution into concentrated sulfuric acid under rapid stirring, and the dropping speed is kept at one drop every two seconds to ensure that the system temperature does not exceed 0°C. After the mixed solution was dripped, the stirring was continued for 12h.
[0059] 4. After the reaction, pour the reaction solution into a large beaker filled with 500mL ice water under rapid stirring, fully dilute the concentrated sulfuric acid in it until the solution is weakly acidic, and a large number of pink flocs will appear in it, filter it.
[0060] 5. Recrystallize the solid obtained by filtration with ethanol / water to obta...
Embodiment 2
[0077] Embodiment 2: Preparation of 4-trifluoromethyl-7-allyloxycoumarin (b-1~4)
[0078] 1. Take 2.3g (0.01mol) 4-trifluoromethyl-7-hydroxycoumarin (a-1) in a round bottom flask, add 3.5g (0.025mol) K 2 CO 3 , 0.33g (0.001mol) tetrabutylammonium bromide, and 30mL acetone, make it mix evenly under magnetic stirring, then add 0.02g KI as catalyst;
[0079] 2. The temperature of the mixed solution was raised to 60° C. under stirring, and 1.3 mL (0.015 mol) of allyl bromide was added after 15 minutes, and then stirred and refluxed for 6 hours, and the progress of the reaction was monitored by TCL.
[0080] 3. After the reaction is completed, filter to remove insoluble matter such as potassium carbonate, spin and concentrate to obtain crude product b-1, and recrystallize with ethanol / water to obtain compound b-1, 4-trifluoromethyl-7-allyloxy incense Soybean.
[0081]
[0082] White crystal m.p.: 120.4-121.5°C; yield: 87.4%.
[0083] IR(KBr)ν / cm -1 :2933,1719,1612,1390,1286...
Embodiment 3
[0097] Example 3: Synthesis of 4-trifluoromethyl-7-prenyloxycoumarin derivatives (c-1~4)
[0098] 1. Take 2.3g (0.01mol) 4-trifluoromethyl-7-hydroxycoumarin in a round bottom flask, add 3.5g (0.025mol) K 2 CO 3 , 0.33g (0.001mol) tetrabutylammonium bromide, and 30mL acetone, make it mix evenly under magnetic stirring, then add 0.02g KI as catalyst;
[0099] 2. The temperature of the mixed solution was raised to 60°C under stirring, and 1.7 mL (0.015 mol) of bromoisoamylene was added after 15 minutes, and then stirred and refluxed for 6 hours, and the progress of the reaction was monitored by TCL.
[0100] 3. After the reaction is completed, filter to remove insoluble matter such as potassium carbonate, spin and concentrate to obtain crude product c-1, and recrystallize with ethanol / water to obtain compound c-1,4-trifluoromethyl-7-prenyloxy Coumarin.
[0101] Compounds c-2~4 are synthesized according to the method similar to c-1, the difference is that 4-trifluoromethyl-7-hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com