Preparation method of empagliflozin
An empagliflozin and reaction technology, applied in the field of medicinal chemistry, can solve the problems of expensive raw materials and high cost, and achieve the effects of improving purity, less impurities and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
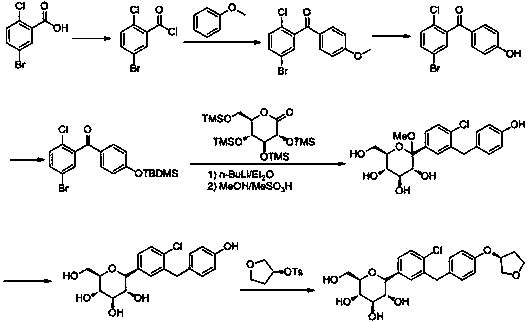
[0039] The preparation method of empagliflozin of the present invention uses 2-chlorobenzaldehyde as the starting material, undergoes bromination, reduction, halogenation, and (S)-3-phenoxytetrahydrofuran for Friedel-Grams alkylation reaction to obtain Intermediate (S)-3-(4-(5-bromo-2-chlorobenzyl)phenoxy)tetrahydrofuran, followed by 2,3,4,6-tetra-O-trimethylsilyl-D- Gluconolactone is condensed, etherified, and demethoxylated to obtain the hypoglycemic drug empagliflozin; specifically, it includes the following steps:
[0040] (1) Preparation of (S)-3-phenoxytetrahydrofuran (I): Fluorobenzene and (S)-3-hydroxytetrahydrofuran undergo a nucleophilic substitution reaction in a polar solvent. Synthesis of (S)-3-phenoxytetrahydrofuran (I) by reaction; the whole reaction is carried out below 10°C, and the reaction time is 2-3h; the polar solvent can be any one of methanol, ethanol, THF or acetonitrile species, preferably THF; strong base can be potassium tert-butoxide or sodium tert-...
Embodiment 1
[0055] The preparation (I) of embodiment 1 (S)-3-phenoxytetrahydrofuran
[0056] Take 96kg of fluorobenzene, 90kg of (S)-3-hydroxytetrahydrofuran, dissolve them in 300kg of tetrahydrofuran, cool in an ice bath to 0°C, and add dropwise a tetrahydrofuran solution of potassium tert-butoxide (potassium tert-butoxide 120kg, tetrahydrofuran 200kg ) After 30 minutes of dripping, the dropwise addition was completed, and reacted at 5-10°C for 1 hour. After the reaction was completed, 300kg of ice water was added to quench the reaction, and tetrahydrofuran was recovered by distillation under reduced pressure. The residual liquid was extracted by adding 300kg of ethyl acetate, dried over anhydrous sodium sulfate, and filtered , the solvent was recovered from the filtrate, and recrystallized from 70% ethanol to obtain 154 kg of white solid, with a yield of 94%.
Embodiment 2
[0057] The preparation of embodiment 2 5-bromo-2-chlorobenzaldehyde (II)
[0058] Take 140kg of 2-chlorobenzaldehyde, dissolve it in 400kg of dichloromethane, stir under ice bath for 30min, then add 180kg of NBS in batches, keep the temperature of the reaction system below 5°C, after the addition, react for 10h, after the reaction, filter, The filtrate was washed with 300 kg of saturated aqueous sodium bicarbonate solution, and then the organic layer was washed with water until neutral, dichloromethane was recovered, and the residual solid was recrystallized with petroleum ether: ethyl acetate (1:1) to obtain 212 kg of a white solid, with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com