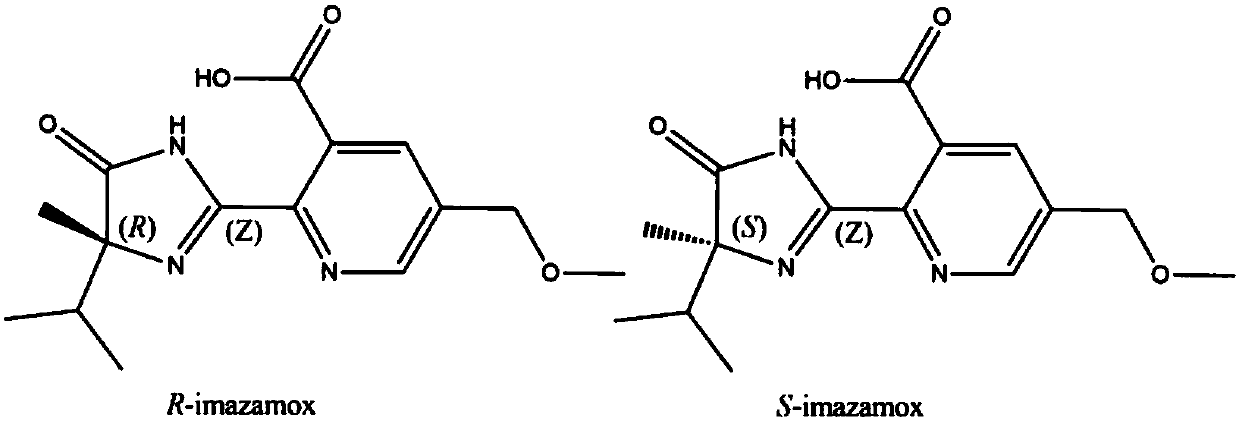
Preparation method and use of R-imazamox
A technology of imazamox and isopropanol, applied in the field of pesticides, can solve the problems of difficult characterization, low enantiomer preparation efficiency and the like, and achieve the effect of wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further elaborated and illustrated below in conjunction with the accompanying drawings and specific embodiments. The technical features of the various implementations in the present invention can be combined accordingly on the premise that there is no conflict with each other.
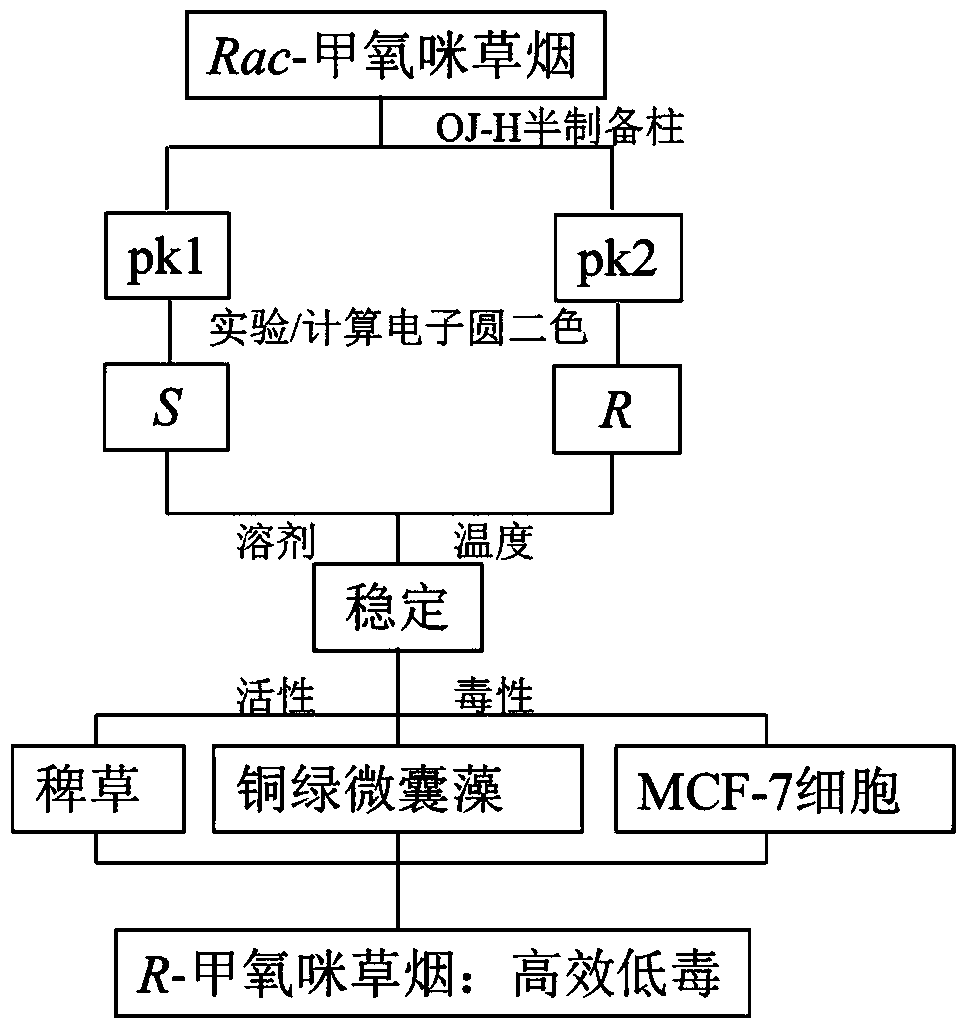
[0025] like figure 2 As shown, in this embodiment, the method for screening and preparing the high-efficiency and low-toxic imazethapyr enantiomer and its absolute configuration characterization includes the following steps:
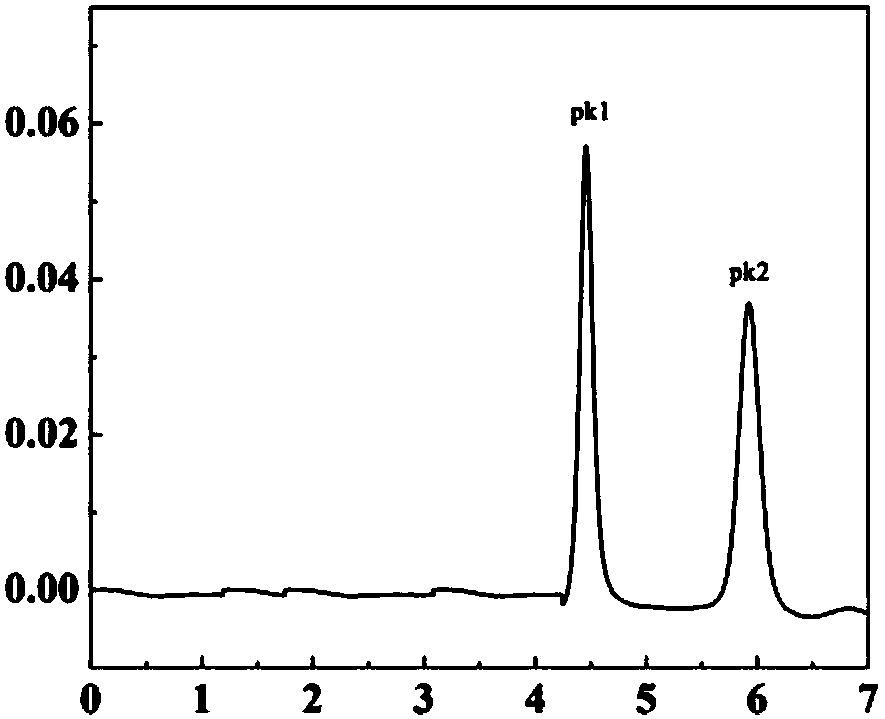
[0026] 1) Dissolve Rac-imazethapyr in isopropanol to prepare 1000mg L -1 For the stock solution, use the semi-preparative chiral column OJ-H column to optimize the mobile phase, column temperature, flow rate and other conditions of high performance liquid chromatography, and balance the relationship between resolution and preparation time. Finally, n-hexane / isopropanol / trifluoroacetic acid (volume ratio 50 / 50 / 0.1) was used as the mobile pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com