High specific surface area catalyst, preparation method and applications thereof
A technology with high specific surface area and specific surface area, which can be used in the preparation of dehydrohalogenation, chemical instruments and methods, catalysts for physical/chemical processes, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
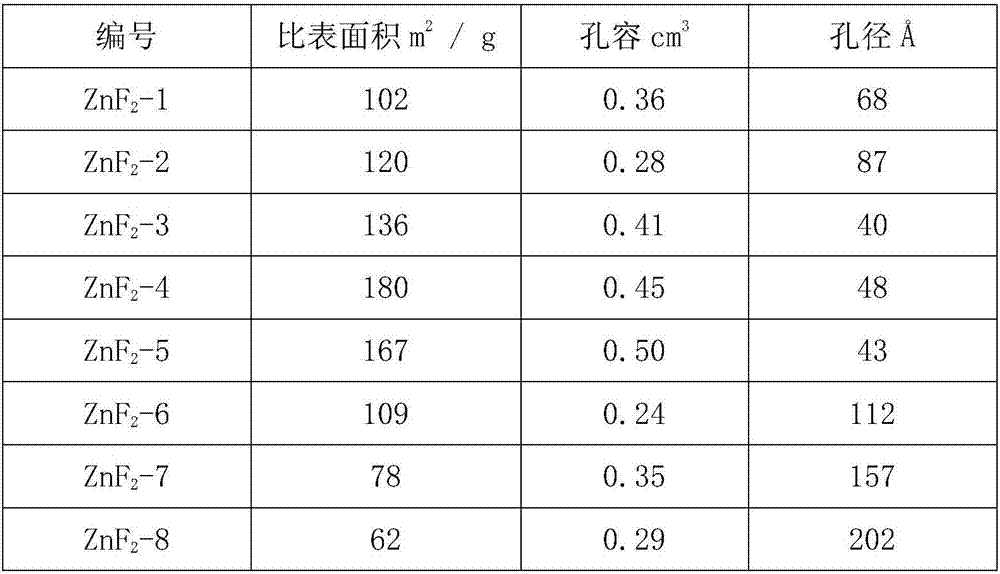
[0032] Take 39.54g Zn(CH 3 COO) 2 Dissolve in 500ml of methanol and stir to form a zinc solution. At room temperature, dissolve 10.0 g of anhydrous HF in HF-Et 2 O solution (12.5mol / L) was added to the above zinc solution, stirred vigorously, stopped stirring after 4 hours of reaction, and left to age for 4 hours. Use a rotary evaporator or a centrifuge to remove the volatile components in the product, and dry them in a vacuum oven to obtain zinc fluoride with a high specific surface area, which is recorded as ZnF 2 -1.
Embodiment 2
[0034] Take 51.08g Zn(MeO) 2 Dissolve in 500ml of methanol and stir to form a zinc solution. At room temperature, dissolve 20.0 g of anhydrous HF in HF-Et 2 O solution (12.5mol / L) was added to the above zinc solution, stirred vigorously, stopped stirring after 4 hours of reaction, and left to age for 4 hours. Use a rotary evaporator or a centrifuge to remove the volatile components in the product, and dry them in a vacuum oven to obtain zinc fluoride with a high specific surface area, which is recorded as ZnF 2 -2.
Embodiment 3
[0036] Take 52.72g Zn(CH 3 COO) 2 Dissolve in 500ml of methanol and stir to form a zinc solution. At room temperature, dissolve 10.0 g of anhydrous HF in HF-Et 2 O solution (12.5mol / L) was added to the above zinc solution, stirred vigorously, stopped stirring after 4 hours of reaction, and left to age for 4 hours. Use a rotary evaporator or a centrifuge to remove the volatile components in the product, and dry them in a vacuum oven to obtain zinc fluoride with a high specific surface area, which is recorded as ZnF 2 -3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com