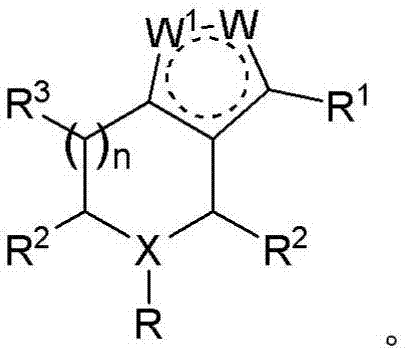
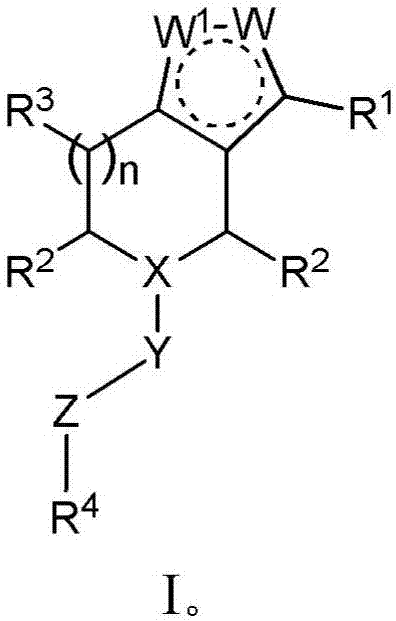
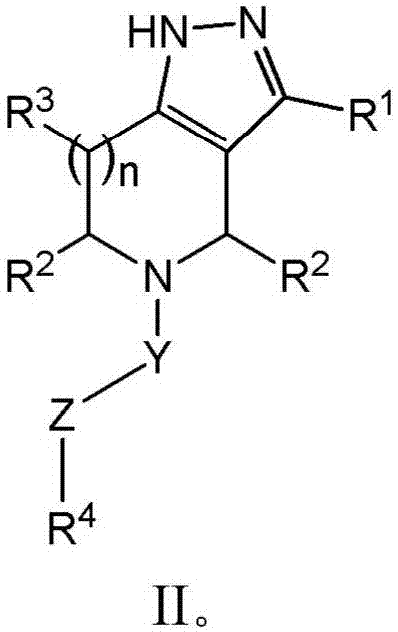
Derivatives and methods of treating hepatitis B infections
A compound and alkyl technology, applied in the field of derivatives and methods for treating hepatitis B infection, can solve the problems of inability to provide cure, difficult complete inhibition, low cure rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0317] Preparation of the optically active form is accomplished in any suitable manner, including, by way of non-limiting examples, resolution of the racemic form by recrystallization techniques, synthesis from optically active starting materials, chiral synthesis, or use of a chiral stationary phase Perform chromatographic separation. In one embodiment, mixtures of one or more isomers are used as therapeutic compounds described herein. In another embodiment, the compounds described herein contain one or more chiral centers. These compounds are prepared by any means including stereoselective synthesis, enantioselective synthesis and / or separation of enantiomeric and / or diastereomeric mixtures. Resolution of compounds and isomers thereof is achieved by any means including, by way of non-limiting examples, chemical methods, enzymatic methods, fractional crystallization, distillation and chromatography.
[0318] In one embodiment, the compounds of the invention may exist as tau...
Embodiment 1
[0394] Example 1: Preparation of Compounds 010 and 059
[0395]
[0396] Step 1: Preparation of compound 3
[0397] at 30°C and N 2 3-Phenyl-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridine (1.00 g, 4.24 mmol, 1.00 equiv) and 2-phenoxyacetic acid ( A mixture of 645.50 mg, 4.24 mmol, 1.00 equiv) in DCM (40 mL) was added in one portion with HATU (1.93 g, 5.09 mmol, 1.20 equiv) and DIPEA (1.37 g, 10.60 mmol, 2.50 equiv). The mixture was stirred at 30°C for 12 hours. LCMS showed the reaction was complete. The mixture was poured into water (50 mL) and stirred for 2 min. The aqueous phase was extracted with DCM (20 mL*2). The combined organic phases were washed with saturated brine (20mL*2), washed with anhydrous Na 2 SO 4Dry, filter and concentrate in vacuo. The residue was purified by silica gel chromatography (petroleum ether / ethyl acetate=3 / 1) to give 2-phenoxy-1-(3-phenyl-1,4,6,7-tetrahydro as a yellow solid Pyrazolo[4,3-c]pyridin-5-yl)ethanone (1.30 g, 3.90 mmol, ...
Embodiment 2
[0400] Embodiment 2: the preparation of compound 011 and 060
[0401]
[0402] at 0°C and N 2 , to 2-phenoxy-1-(3-phenyl-1,4,6,7-tetrahydropyrazolo[4,3-c]pyridin-5-yl)ethanone (150.00mg, 449.94 umol, 1.00 equiv) in DMF (10 mL) was added NaH (36.00 mg, 899.88 umol, 2.00 equiv) in one portion. The mixture was stirred at 0°C for 0.5 hours, then ethyl iodide (210.53 mg, 1.35 mmol, 3.00 equiv) was added to the mixture at 0°C. The mixture was heated to 30°C and stirred for 12 hours. LCMS showed the reaction was complete. The mixture was quenched with water (5 mL) and stirred for 5 min. The aqueous phase was extracted with ethyl acetate (15 mL*2). The combined organic phases were washed with saturated brine (15mL*2), washed with anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. The residue was purified by preparative HPLC (FA) to give 1-(1-ethyl-3-phenyl-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine as a white solid -5-yl)-2-phenoxy-ethanone (71.20mg, 196.99umol, 43.78...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com