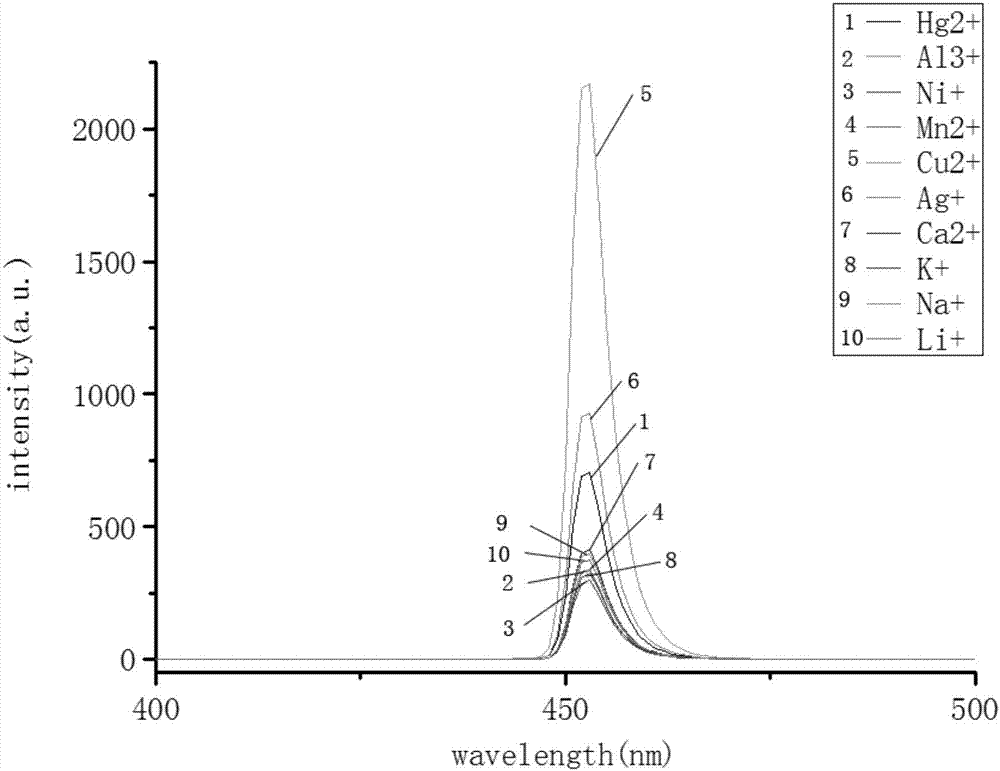
2-arylimidazole-phenanthroline probe capable of recognizing cu<2+> in HL-60 and preparation method thereof
A technology of aryl imidazolo and o-phenanthroline, which is applied in the field of fluorescent probes for metal ions, can solve problems such as life safety threats, acute illness, and dangerous prognosis, and achieve high sensitivity, high response value, and good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: This embodiment is a specific embodiment prepared according to the reaction scheme of the compound of formula (I).
[0032] Phenylimidazo[5,6-f]phenanthroline: Weigh 2.0g of 1,10-phenanthroline and 6.0g of selenium dioxide, mix them evenly in a flask, and make 20 mL of 18mol·L -1 Concentrated sulfuric acid, 10 mL concentration is 14mol•L -1Concentrated nitric acid is mixed and cooled in an ice-water bath to form a cooled mixed acid. Slowly add the cooled mixed acid dropwise along the wall of the flask. After the dropwise addition, heat to reflux, and a reddish-brown gas is gradually generated in the bottle. Stop heating to obtain a reddish-brown transparent solution; pour the reddish-brown transparent solution into ice water, and slowly neutralize the acid with NaOH until the pH of the reaction mixture is 7. During this process, many yellow flocculent precipitates are formed, and the yellow precipitate is obtained by suction filtration , extracted three ...
Embodiment 2
[0034] Embodiment 2: 2-(2-hydroxyl-5-nitrophenyl) imidazo[5,6-f] o-phenanthroline: the preparation method is the same as in Example 1, adding 5-nitrosalicylaldehyde to obtain light Yellow needle-point crystals, yield 57%. Its hydrogen nuclear magnetic resonance spectrum data are:
[0035] 1 H NMR (500 MHz, DMSO-d6): 9.06 (d, 2H, J = 8.2 Hz), 8.98 (d, 2H, J = 7.5 Hz), 7.87 (dd, 2H, J = 8.0 Hz), 7.43 (d ,1H, J =8.0 Hz), 6.84 (d, 1H, J =8.6 Hz),6.73—6.75 (m, 1H).
Embodiment 3
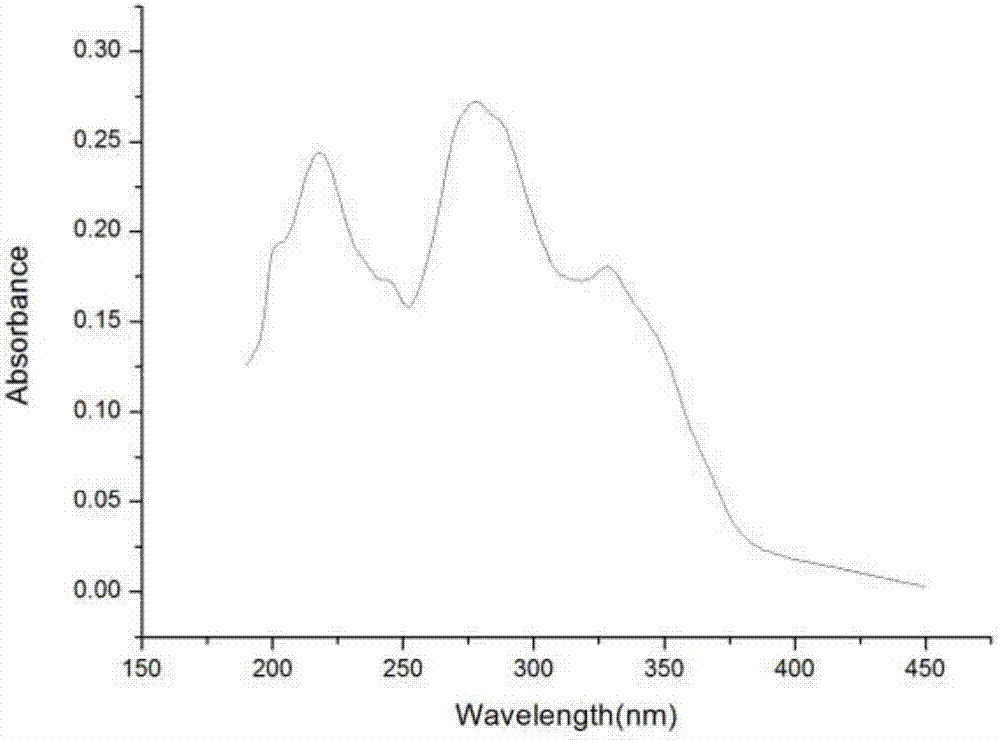
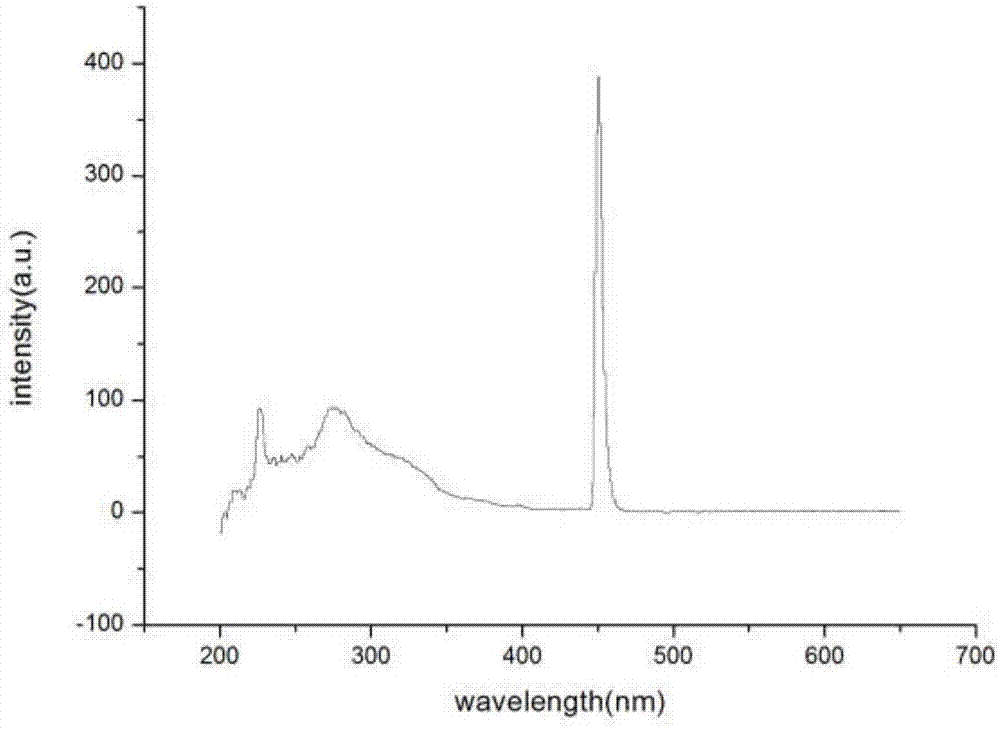
[0036] Embodiment 3: the ultraviolet absorption spectrum of the 2-(2-hydroxyl-5-nitrophenyl) imidazo[5,6-f] o-phenanthroline fluorescent probe that embodiment 2 makes (see figure 1 ) and fluorescence spectra (see Picture 1-1 ).
[0037] Accurately weigh 2-(2-hydroxy-5-nitrophenyl)imidazo[5,6-f]phenanthroline, transfer it into a 10 mL volumetric flask, dissolve it with absolute ethanol, and wait until the sample is completely dissolved After constant volume, prepare to a concentration of 1.0×10-5 mol•L -1 The solution was measured for its UV absorption spectrum and fluorescence excitation spectrum, respectively. Measurement results such as figure 1 , Picture 1-1 shown; figure 1 is the ultraviolet absorption spectrum of copper ion fluorescent probe 2-(2-hydroxyl-5-nitrophenyl)imidazo[5,6-f]phenanthroline; Picture 1-1 It is the fluorescence spectrum of the copper ion fluorescent probe 2-(2-hydroxyl-5-nitrophenyl)imidazo[5,6-f]phenanthroline for the detection of aggregati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com