5-(benzofuran-2-carbonyl)-6-carboxamide-5,6-dihydro-phenanthridine derivative and synthesis and application thereo
A technology of benzofuran and dihydrophenanthridine, which is applied in the field of 5--6-carboxamide-5, can solve the problems that have not been reported, and achieve the effects of low preparation cost, high research value, and efficient synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
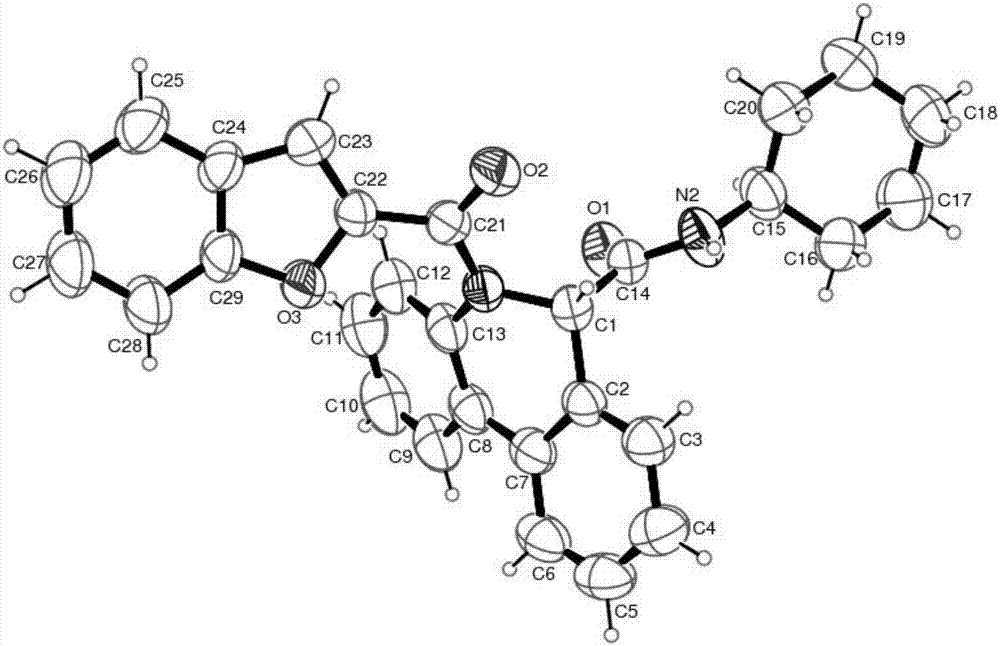
[0030] 5-(benzofuran-2-carbonyl)-6-carboxamide-5,6-dihydrophenanthridine derivatives, the chemical structure of which is shown in formula VI:
[0031]
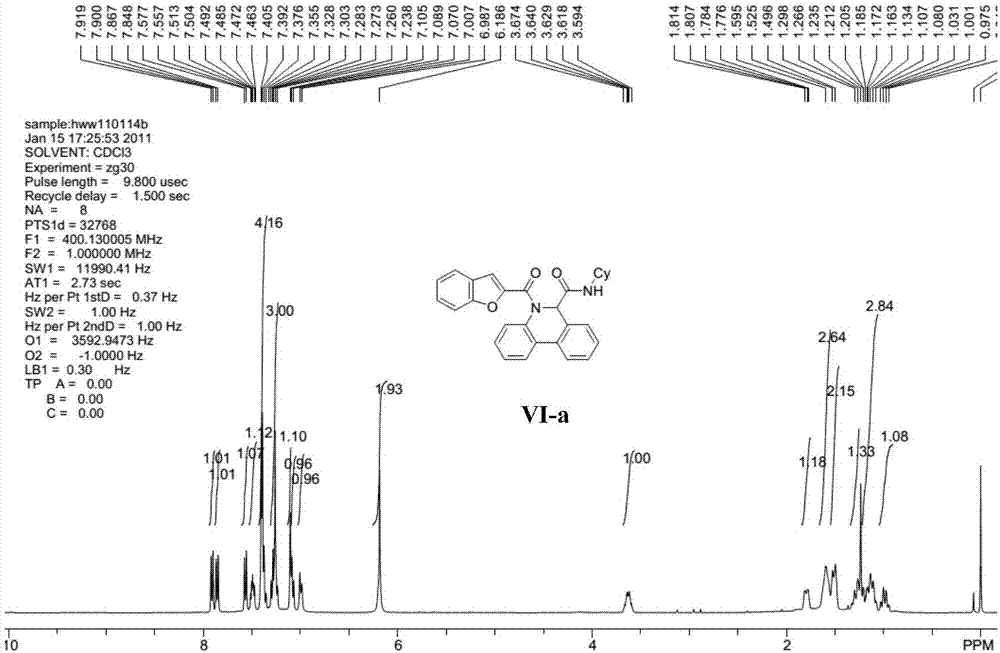
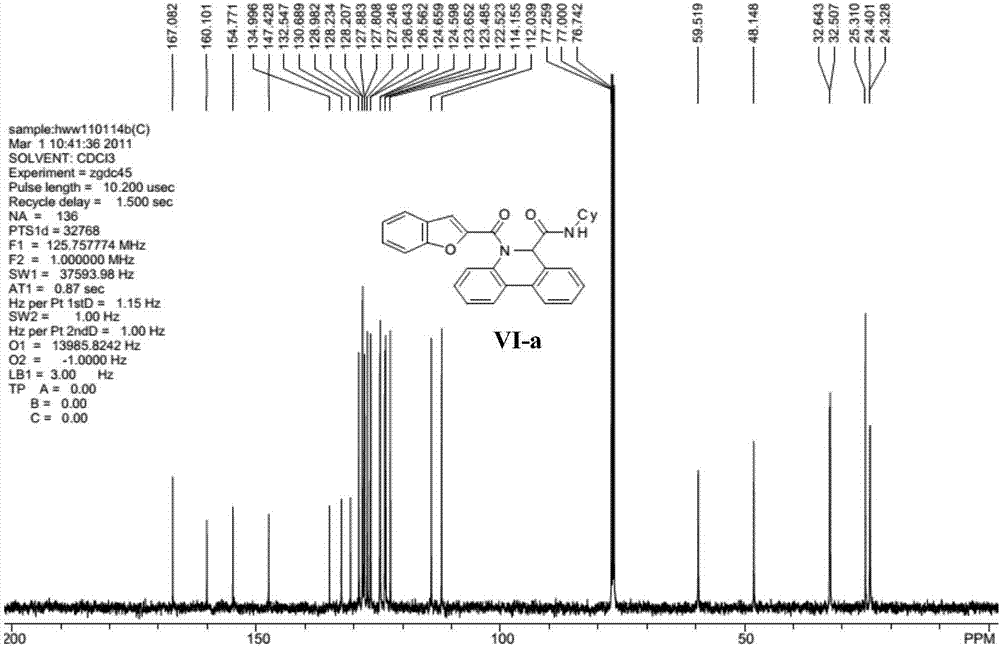
[0032] Representative compound VI-a (R in the formula VI 1 is hydrogen; R 2 is cyclohexyl; R 3 For hydrogen) the synthetic steps are as follows:
[0033]
[0034] Benzofuran-2-carboxamide (0.20mmol) and palladium acetate (10mol%), tricyclohexylphosphine fluoroborate (20mol%), pivalic acid (PivOH) (30mol%) and potassium carbonate (0.40mmol%) ) into the microwave tube, then cover the lid, pump nitrogen three times on the oil pump. Pour degassed anhydrous N,N-dimethylacetamide (4mL) into the microwave tube, heat the reaction in an oil bath to 110°C and stir for 4 hours. Filter while washing with ethyl acetate. The filtrate was dried with anhydrous sodium sulfate, concentrated, and separated by column chromatography with ethyl acetate and petroleum ether (ethyl acetate / petroleum ether=1 / 10) to obtain the target compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com