Pheochromocytoma diagnostic kit and using method thereof
A technology for pheochromocytoma and diagnostic kits, applied in the field of providing a kit, which can solve the problems of high false positive rate and achieve the effect of simple reagents, low price and high diagnostic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of pheochromocytoma diagnostic kit
[0045] 1. Instruments and equipment
[0046] 1. Analytical balance: Sensitivity is 0.0001g
[0047] 2. Microporous membrane: pore size 0.22μm
[0048] 3. Agilent 1100 High Performance Liquid Chromatograph
[0049] 4. Millipore ultrapure water filtration device and Millipore vacuum pump (Millipore Corporation, USA)
[0050] 5. HIMAC-cf 15 low temperature desktop centrifuge
[0051] 2. Reagents and solutions: the components of the kit are shown in Table 2
[0052] Table 2 Kit components
[0053]
[0054] The preparation method of the above-mentioned kit specifically includes:
[0055] (1) Sample pretreatment reagents:
[0056] ①Prepare 10% ammonia water methanol solution (volume ratio 3:1); filter with 0.22 μm organic membrane, and store in 4°C refrigerator for later use.
[0057] ② Prepare 0.18mol / L potassium hydroxide methanol solution; filter with 0.22μm organic membrane, and store in 4°C ref...
Embodiment 2
[0065] Example 2: Use of Pheochromocytoma Diagnostic Kit
[0066] 1. Urine Sample Pretreatment
[0067] Take 500μL random urine, add 5mL ultrapure water, 200μL 0.2mol / L acetic acid, and mix well. Use 5mL of 10% ammonia water-methanol (volume ratio 1:3), 2mL of 0.18mol / L potassium hydroxide methanol solution, and 2mL of ultrapure water to activate the solid-phase extraction column in turn, mix the above samples and load them, and control the flow rate to 1 -2mL / min. Rinse the cartridge sequentially with 4mL 10mmol / L acetic acid-methanol (volume ratio 9:1), 4mL 10mmol / L ammonium phosphate, and 4mL ultrapure water. 2mL ammonia water-methanol solution was used for elution, the eluate was spin-dried in vacuum and then redissolved with 200μL 0.20mol / L acetic acid solution.
[0068] 2. Liquid chromatography conditions
[0069] Mobile phase: mobile phase A is 70mmol / L NaH 2 PO 4 solution, the mobile phase B is methanol (chromatographically pure), and a gradient elution method is...
Embodiment 3
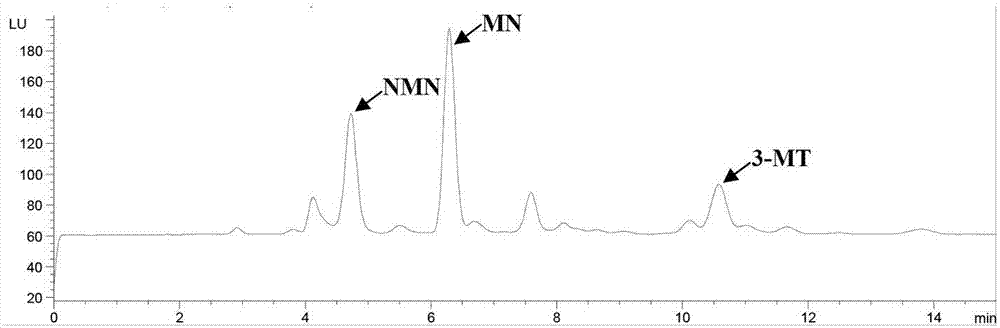
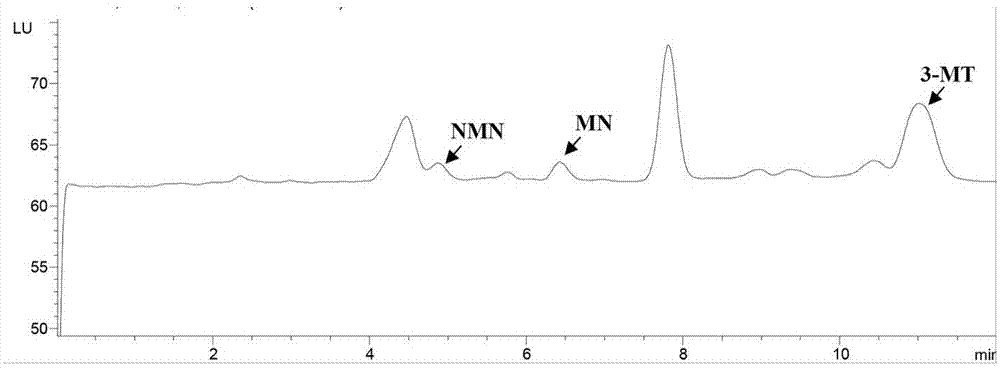
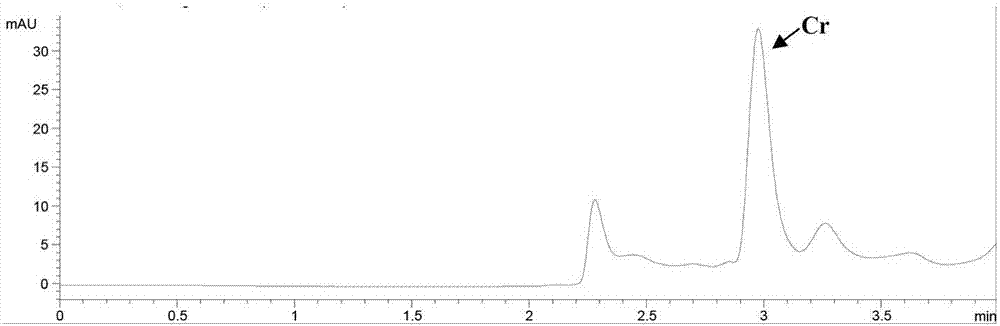
[0078] This example is to investigate the precision of the kit of the present invention for determining NMN, MN and 3-MT in random urine. Real samples were used to investigate the precision, that is, the urine of patients with pheochromocytoma and the urine of normal people were selected, and the measurement was repeated 5 times a day for 5 consecutive days, and the ratio of the analyte to creatinine was calculated to evaluate the precision. See Table 4. The coefficients of variation are all equal to or less than 5.6%, indicating that the present invention has good precision.
[0079] Table 4 Precision (n=5)
[0080]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com