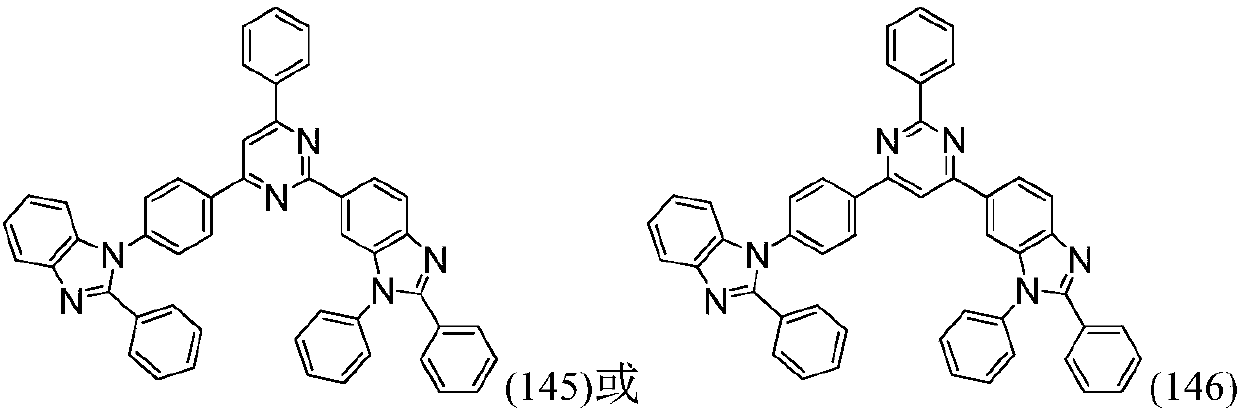
Organic compound based on pyridine and benzimidazole and application thereof in organic light emitting diodes (OLED)
An organic compound and benzimidazole technology, applied in the field of organic compounds and its application in OLED, can solve the problems affecting the angular distribution of OLED radiation spectrum and complex manufacturing process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the synthesis of intermediate I
[0068] When azabenzene and Ar 1 When connected by C-C bond,
[0069]
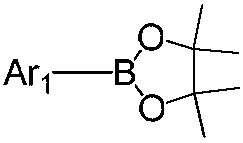
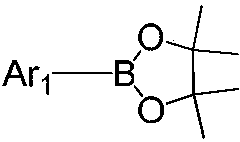
[0070] Under nitrogen atmosphere, weigh Ar 1 The bromide was dissolved in tetrahydrofuran (THF), and then bis(pinacolyl)diboron, (1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium(II) and potassium acetate were added, The mixture is stirred, and the mixed solution of the above reactants is heated to reflux at a reaction temperature of 70-90° C. for 5-10 hours; after the reaction is completed, water is added to cool, and the mixture is filtered and dried in a vacuum oven. The obtained residue was separated and purified by silica gel column to obtain Ar 1 pinacol borate;
[0071]
[0072] Under a nitrogen atmosphere, weigh raw material A and dissolve it in N,N-dimethylformamide (DMF), and then and palladium acetate, stir the mixture, then add potassium phosphate aqueous solution, heat the mixed solution of the above reactants to reflux at...
Embodiment 2
[0095] Embodiment 2: intermediate Synthesis
[0096] When R 1 or R 2 When expressed as a structure of general formula (6),
[0097]
[0098] (1) In a 250mL three-necked flask, feed nitrogen, add 0.02mol raw material 2-bromo-benzimidazole, 0.03mol I-Ar 4 , 0.04mol sodium hydride, 0.004mol cuprous iodide and 0.01mol o-phenanthroline were dissolved in 100ml 1,3-dimethyl-2-imidazolidinone, stirred and reacted for 20-30h, after the reaction, add water and use two Extract with methyl chloride, dry the organic layer with anhydrous sodium sulfate, rinse with a mixture of petroleum ether and ethyl acetate as eluent, the volume ratio of petroleum ether and ethyl acetate in the eluent is 1:100, and purify by column chromatography , to obtain intermediate M;
[0099]
[0100] (2) Under a nitrogen atmosphere, weigh the intermediate M and dissolve it in tetrahydrofuran, and then dissolve the Br-Ar 2 -B(OH) 2 And tetrakis (triphenylphosphine) palladium is added, the mixture is ...
Embodiment 3
[0132] Embodiment 3: the synthesis of compound 3:
[0133]
[0134] In a 250mL three-neck flask, blow nitrogen, add 0.01mol intermediate A1, 150ml DMF, 0.03mol intermediate B1, 0.0002mol palladium acetate, stir, and then add 0.02mol K 3 PO 4 The aqueous solution was heated to 150°C, refluxed for 24 hours, sampled and plated, and the reaction was complete. Cool naturally, extract with 200ml of dichloromethane, separate layers, dry the extract with anhydrous sodium sulfate, filter, rotate the filtrate, and purify through a silica gel column to obtain the target product with a HPLC purity of 99.4% and a yield of 64.7%.
[0135] Elemental analysis structure (molecular formula C 55 h 37 N 5 ): theoretical value C, 86.02; H, 4.86; N, 9.12; test value: C, 86.05; H, 4.85; N, 9.10. ESI-MS(m / z)(M + ): The theoretical value is 767.30, and the measured value is 767.58.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com