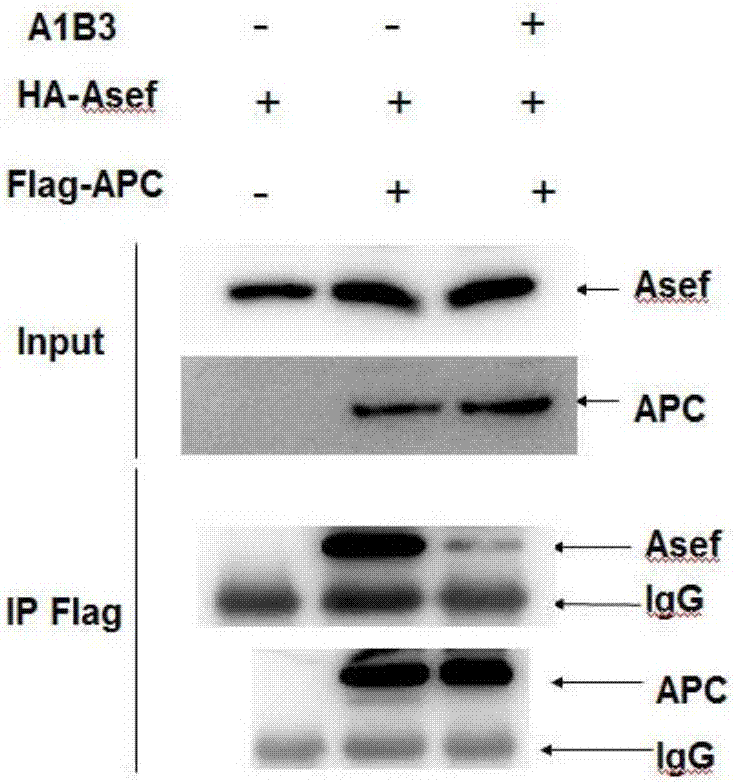
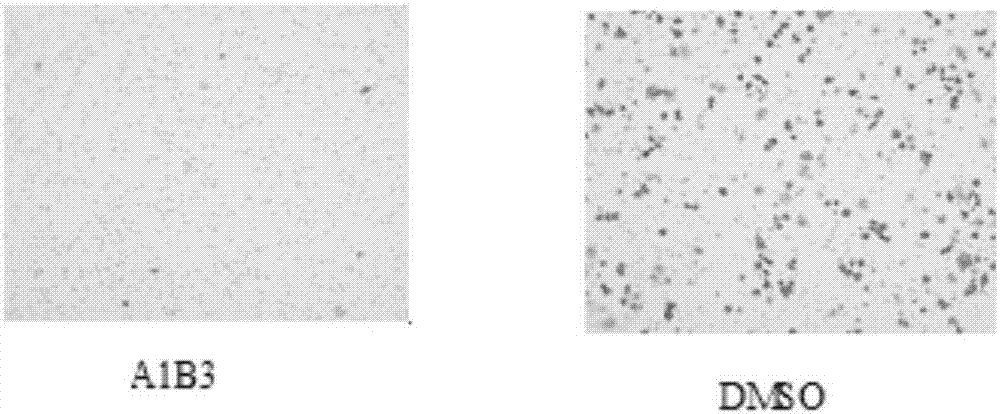
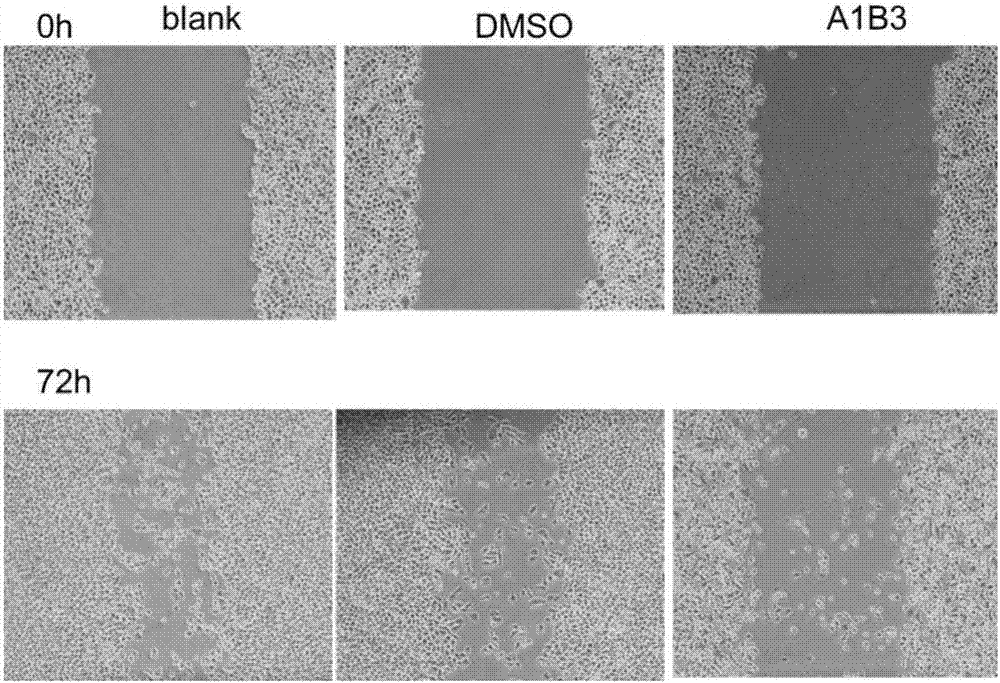
Polypeptide and application thereof
A representative and sequence technology, applied in the field of anti-tumor polypeptide and its application, can solve the problems of limited application, full length of APC, difficult transfection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Solid phase synthesis, cleavage, purification and identification of polypeptide 1
[0047] 1.1 Solid phase synthesis of polypeptide 1
[0048] The present invention utilizes a polypeptide synthesizer to synthesize polypeptide 1 (Ac-AGEALYE-NH2) by solid-phase synthesis: using DMF as a solvent, the concentration of various α-amino acids protected by Fmoc is 0.25M, the concentration of HBTU solution and HOBt solution is 0.33M, the concentration of piperidine solution is 200ml / L, and the concentration of DIEA solution is 174.2ml / L.
[0049] 1.1.1 Fmoc protecting group
[0050] Take 1g of Rink Amide MBHA Resin (the degree of substitution of the resin is 0.40mmol / g, and the resin is protected by Fmoc) into the peptide bottle, add an appropriate amount of CH 2 Cl 2 Swell the resin, then put the CH 2 Cl 2 Take out and add 6ml 20% piperidine / DMF solution, shake for 5 minutes, drain, then add 6ml 20% piperidine / DMF solution, shake for 15 minutes, then drain, wash ...
Embodiment 2
[0059] Example 2 Prepare 5 mg of polypeptide 2Ac-AGEAIYE-NH2 according to the same method as in Example 1, ESI: 791.8 [M-H] - , HPLC: 98.3%.
Embodiment 3
[0060] Example 3 Prepare polypeptide 3Ac-AGESLYE-NH2 Ac-AGEAIYE-NH2 5 mg according to the same method as Example 1, ESI: 807.9 [M-H] - , HPLC: 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com