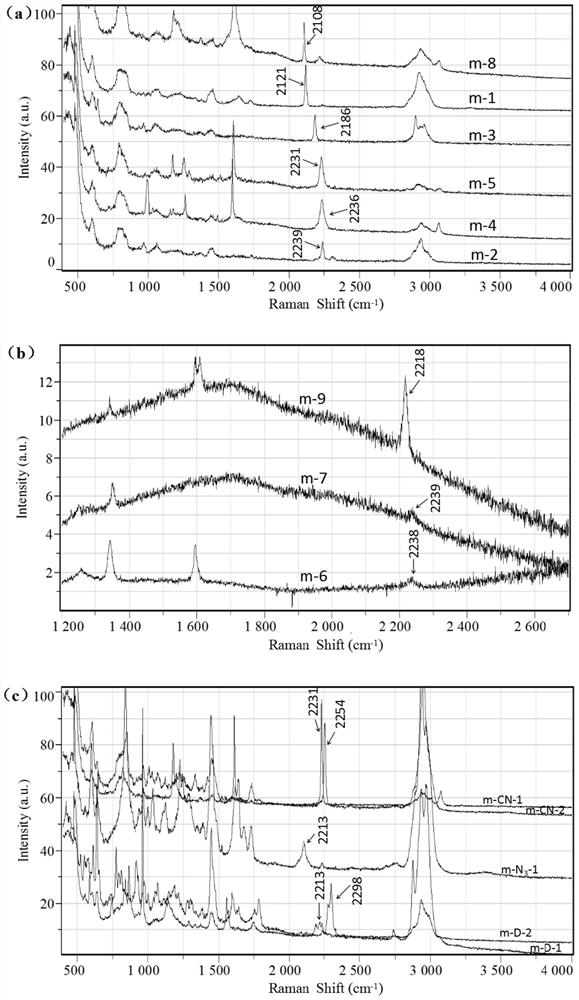
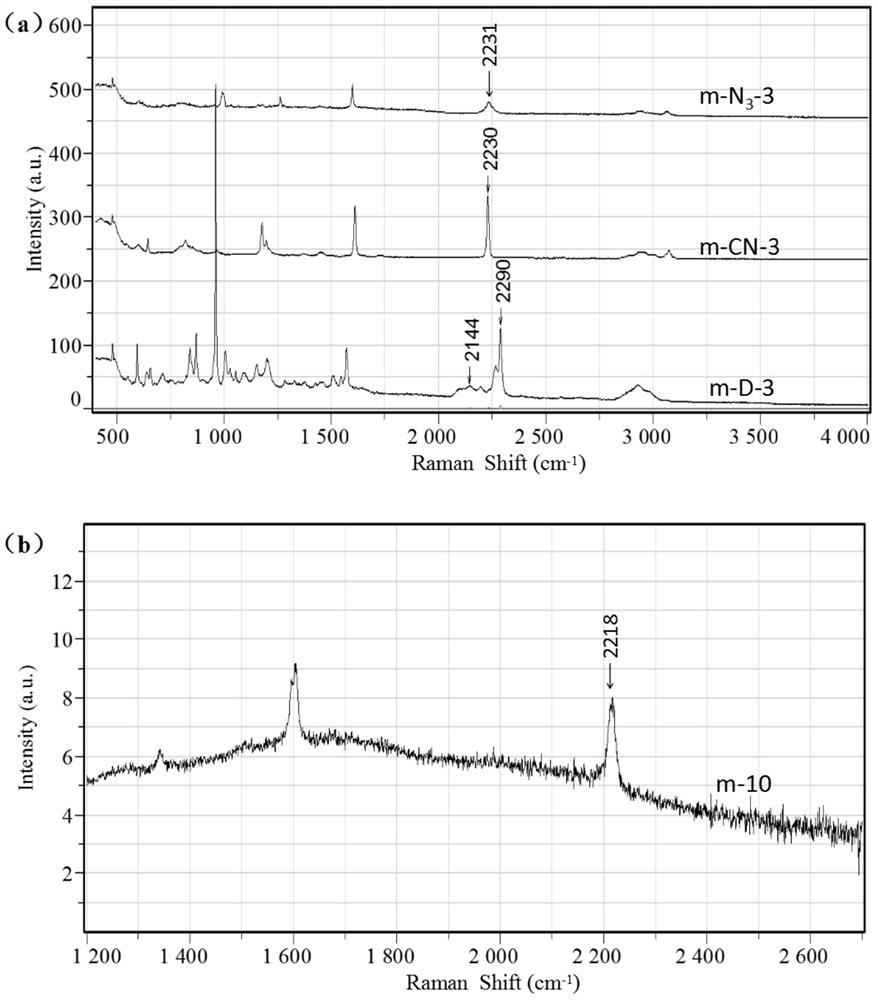
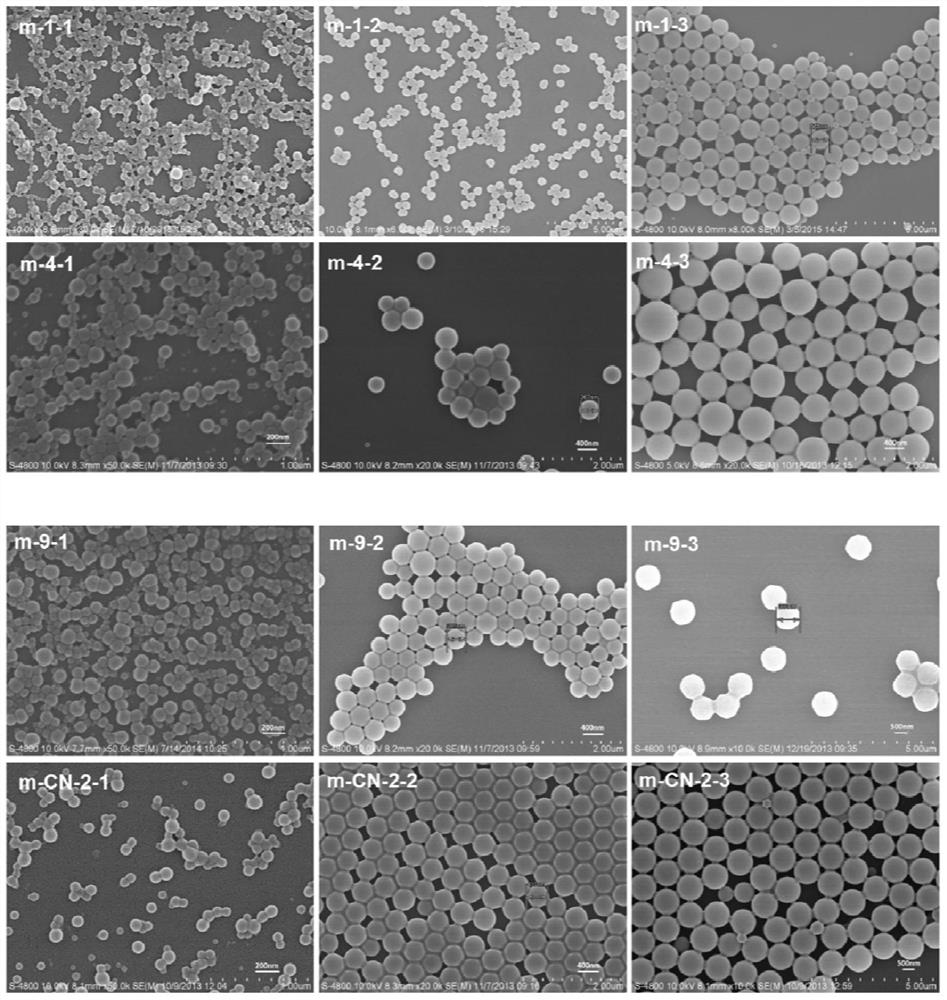
Application of a polymer microsphere in Raman detection
A technology of polymers and amide polymers, which is applied in the application field of polymer microspheres in the field of bioimaging, and can solve problems such as poor reproducibility and difficult quantitative analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1. Synthesis of compound m-1 monomer
[0053]
[0054] The raw material butynyl alcohol (700 mg, 10 mmol) was dissolved in 20 mL of anhydrous DCM, 4.15 mL (30 mmol) of dry TEA was added, and N 2 For protection, methacryloyl chloride (1.65 mL, 17 mmol) was added dropwise to the solution under ice-cooling. After the dropwise addition was completed, it was moved to normal temperature for 6 h. The reaction solution was extracted with DCM, saturated NaHCO 3 The solution was washed twice, and the saturated NaCl solution was washed once. Anhydrous Na for organic phase 2 SO 4 Dry, filter, concentrate, and use PE and DCM as eluents (volume ratio 1:2) for column purification to obtain 940 mg of monomer m-1 as a colorless oily liquid. The yield was 68.1%.
[0055] 1 H NMR (400MHz, CDCl 3 )δ6.14(s,1H),5.58(s,1H),4.25(t,J=6.8Hz,2H),2.57(td,J=6.8,2.7Hz,2H),1.95(s,3H).
[0056] 13 C NMR (101MHz, CDCl 3 )δ167.29, 136.25, 126.04, 80.20, 70.02, 62.49, 19.16, 18.42...
Embodiment 2
[0057] Embodiment 2. the synthesis of compound m-2 monomer
[0058]
[0059] The raw material 2 pentyn-1-ol (840 mg, 10 mmol) was dissolved in 20 mL of anhydrous DCM, 4.15 mL (30 mmol) of dry TEA was added, and N 2 For protection, methacryloyl chloride (1.65 mL, 17 mmol) was added dropwise to the solution under ice-cooling. After the dropwise addition was completed, it was moved to normal temperature for 6 h. The reaction solution was extracted with DCM, saturated NaHCO 3 The solution was washed twice, and the saturated NaCl solution was washed once. Anhydrous Na for organic phase 2 SO 4 It was dried, filtered, concentrated, and purified by column using PE and DCM as eluents (volume ratio 1:2) to obtain 1.23 g of monomer m-2 as a colorless oily liquid. The yield was 80.9%.
[0060] MS[M+Na] + : measured value: 175.2; C 9 h 12 o 2 Theoretical value: 175.08.
[0061] 1 H NMR (400MHz, CDCl 3 )δ6.15(s,1H),6.15(s,1H),5.64–5.54(m,1H),5.62–5.57(m,1H),4.73(s,2H),4.73(s,...
Embodiment 3
[0063] Embodiment 3. the synthesis of compound m-3 monomer
[0064]
[0065] The raw material trimethylsilylpropynol (1.28g, 10mmol) was dissolved in 20mL of anhydrous DCM, 4.15mL (30mmol) of dry TEA was added, and N 2 For protection, methacryloyl chloride (1.65 mL, 17 mmol) was added dropwise to the solution under ice-cooling. After the dropwise addition was completed, it was moved to normal temperature for 6 h. The reaction solution was extracted with DCM, saturated NaHCO 3 The solution was washed twice, and the saturated NaCl solution was washed once. Anhydrous Na for organic phase 2 SO 4 Dry, filter, concentrate, and use PE and DCM as eluents (volume ratio 1:2) for column purification to obtain 1.22 g of monomer m-3 as a colorless oily liquid. The yield was 62.2%.
[0066] 1 H NMR (400MHz, CDCl 3 )δ6.17(s,1H),5.63–5.58(m,1H),4.75(s,2H),1.96(s,3H),0.18(s,9H).
[0067] 13 C NMR (101MHz, CDCl 3 )δ166.70, 135.89, 126.49, 99.27, 92.08, 53.10, 18.42.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com