Chiral alpha-amino acid derivative and preparation method thereof
A technology of chiral amino acids and derivatives, which is applied in the preparation of carbamic acid derivatives, sulfonamides, and organic compounds, and can solve the problems of cumbersome processes, poor applicability, and unsatisfactory catalytic asymmetric synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
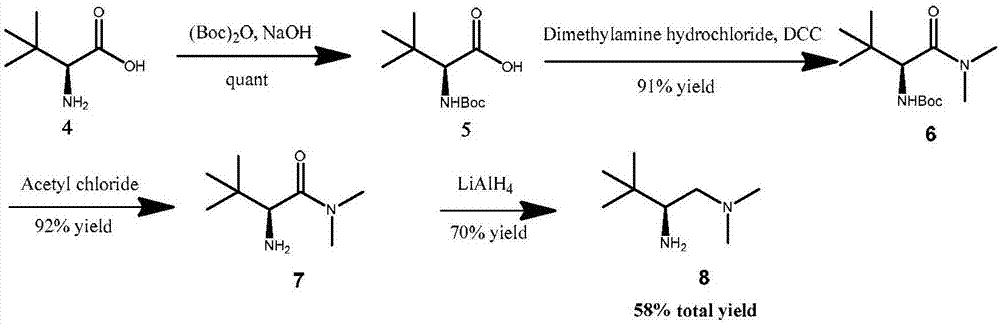
Embodiment 1
[0093] Embodiment 1, N, the preparation of O-acetal
[0094] Method 1: Using CbzNH 2 As the starting material, the target branched chain N, O-acetal compound is obtained through condensation of amine and glyoxylate. The specific synthesis steps are:
[0095] CbzNH 2 (Benzyl carbamate), ethyl glyoxylate are mixed at a molar ratio of 1:1.3, acetic acid and acetic anhydride are mixed at a volume ratio of 1:3 as a reaction solvent, stirred at 60°C for 1 day, and unreacted Acetic anhydride and acetic acid can obtain the corresponding stable branched chain Cbz protected amino N, O-acetal compound, the specific reaction equation is as figure 2 shown;
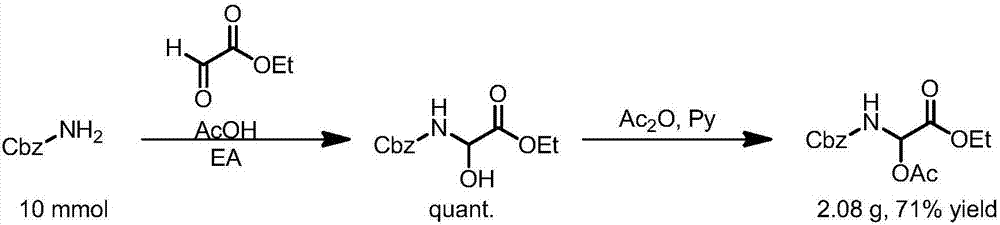
[0096] Method 2: Realized by step-by-step synthesis, first through CbzNH 2 Condensation with glyoxylic acid ester to obtain N, O hemiacetal, and then protect the hydroxyl group with a protecting group, and then obtain the raw material of N, O acetal. The specific method includes the following process steps:
[0097] CbzNH 2 (Benz...
Embodiment 2
[0100] Embodiment 2, N, the preparation of O-acetal
[0101] CbzNH 2 (Benzyl carbamate) and chloral were mixed at a molar ratio of 1:1.3, reacted in ethyl acetate at 60°C for 1 day, and the solvent was removed by rotary evaporation to obtain solid N,O hemiacetal, and then added 5 Molar equivalent of acetic anhydride, 1% molar equivalent of pyridine, react at 25°C for 1 day, remove unreacted acetic anhydride, and obtain branched N,O-acetal compound of Cbz protected amino group, specific reaction equation Such as image 3 shown.
[0102]
[0103] The structure is confirmed as follows: 1 H NMR (400MHz, CDCl 3 )δ7.37(s,5H),6.93(d,J=10.0Hz,1H),5.69(s,1H),5.19(s,2H),2.17(s,3H). 13 C NMR (101MHz, CDCl 3 )δ168.16, 135.51, 128.80, 128.72, 128.53, 98.21, 81.38, 68.26, 20.69.
Embodiment 3
[0104] Embodiment 3, N, the preparation of O-acetal
[0105] CbzNH 2 (Benzyl carbamate), mixed trifluoroacetaldehyde hydrate at a molar ratio of 1:1.3, reacted in ethyl acetate at 60°C for 1 day, and removed the solvent by rotary evaporation to obtain solid N, O hemiacetal, and then added 5 molar equivalents of acetic anhydride, 1% molar equivalents of pyridine, react at 25°C for 1 day, remove unreacted acetic anhydride, and obtain branched N,O-acetal compounds of Cbz protected amino groups, specific reactions Equation such as image 3 shown.
[0106]
[0107] The structure is confirmed as follows: 1 H NMR (500MHz, CDCl 3 )δ7.35(q, J=15.0Hz, 5H), 6.80(s, 1H), 5.66(d, J=9.0Hz, 1H), 5.17(d, J=12.0Hz, 2H), 2.14(s, 3H). 13 C NMR (126MHz, CDCl 3 )δ168.09, 154.27, 135.26, 128.79, 128.75, 128.53, 128.47, 122.80, 120.57, 72.72, 72.43, 72.14, 71.85, 68.32, 20.52.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com