Application of compound SS-31 to prepare medicine for treating Friedreich's ataxia and related diseases
A technology of SS-31, compound, applied in the field of application in the treatment of Friedreich's ataxia disease, to achieve the effect of huge market value and social benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
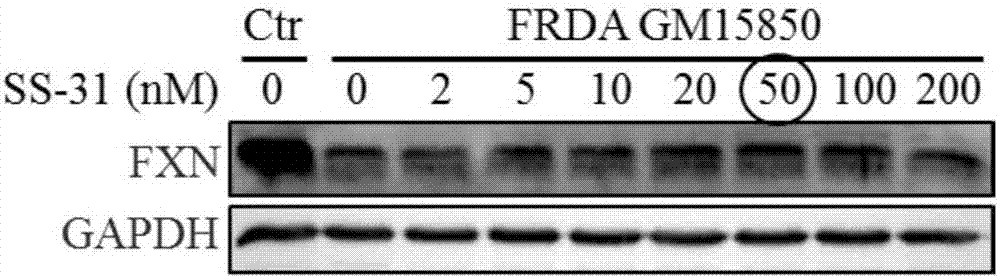
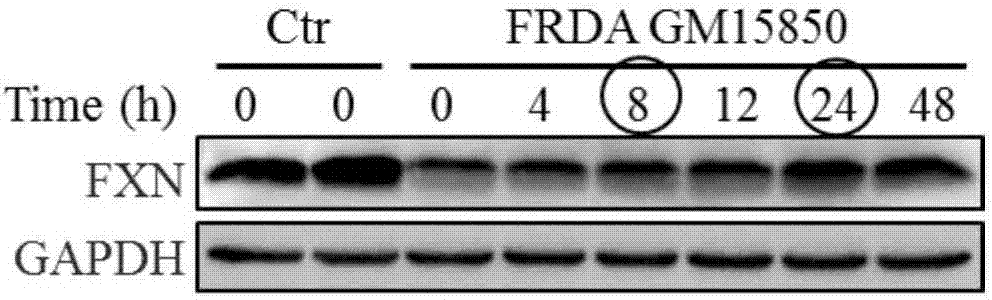
[0058] Example 1 The optimization of SS-31 administration concentration and time is conducive to improving the expression of FXN in FRDA patient cells
[0059] 1.1 Experimental materials
[0060] Cells: lymphocyte line GM15850 derived from FRDA patients, normal human lymphocyte line GM15849. Culture conditions: culture in 1640 medium containing 10% fetal bovine serum (FBS), 2mM glutamate, and 100U / mL penicillin and streptomycin at 37°C and 5% CO2.
[0061] SS-31 was synthesized by Shanghai Qiangyao Biotechnology Co., Ltd., dissolved in PBS, stored at a concentration of 1mM, and stored at -80°C.
[0062] FXN antibody was obtained by immunization in our laboratory, and GAPDH antibody was purchased from Abgent.
[0063] 1.2 Experimental method
[0064] 1.1.1 Treat the patient's cells with 0, 2, 5, 10, 20, 50, 100, 200nM SS-31 respectively, collect the cell pellet and extract the protein after 24 hours. With healthy human cells as the control, after 24 hours of simultaneous cu...
Embodiment 2
[0069] Example 2 SS-31 Treatment Improves the Balance of Patient's Cellular Iron Metabolism
[0070] 2.1 Experimental materials
[0071] IRP2 antibody and TfR1 antibody were obtained by immunization in our laboratory, and Ferritin was purchased from Abcam.
[0072] Calcein-AM was purchased from sigma; RPA (Rhodamine B-[(1,10-phenanthroline-5-yl)-aminocarbonyl]benzyl ester) was purchased from Squarix.
[0073] 2.2 Experimental method
[0074] 2.2.1 First, detect the effect of SS-31 on the level of proteins related to iron metabolism in patients' cells. The patient's cells were treated with 50nm SS-31 for 8 and 24 hours, and the cell pellet was harvested. Western Blotting was used to detect the protein levels of IRP2, TfR1, Ferritin, ISCU, and FXN.
[0075]2.2.2 Secondly, the influence of SS-31 on the content of unstable iron in the patient's cytoplasm and mitochondria was detected. After treating the patient's cells with 50nmSS-31 for 8 and 24 hours, the cells were collecte...
Embodiment 3
[0078] Example 3 Effect of SS-31 treatment on the mitochondrial respiratory chain containing iron-sulfur clusters
[0079] 3.1 Experimental materials
[0080] Mitochondrial Complex I Activity Assay Kit (Abcam)
[0081] Mitochondrial Complex II Activity Detection Kit (Suzhou Keming Biotechnology Co., Ltd.)
[0082] Mitochondrial Complex III Activity Assay Kit (Biovision Inc.)
[0083] Xanthine Oxidase Assay Kit (Nanjing Jiancheng Technology Co., Ltd.)
[0084] 3.2 Experimental method
[0085] 3.2.1 First, the detection of aconitase activity: Prepare 8% separating gel according to the proportion (3.64mL of double distilled water, 0.94mL of 10×TB buffer solution, 1.68mL of 30% Acr-Bis, 22.5μL of 1M sodium citrate , 10% APS 31 μL, TEMED 6.25 μL), and mix the solution evenly. Take 5mL of separation gel and pour it into the gel-making device, then add 1mL of 50% ethanol to seal the liquid surface of the gel, pour off the ethanol after the separation gel is solidified, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com