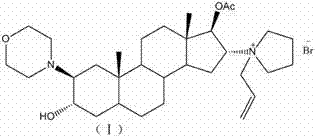
Novel method for preparing rocuronium bromide
A synthesis method, the technology of pyrrole bromide, applied in the field of chemical pharmacy, can solve the problems of poor chemical selectivity, achieve high purity, easy post-processing purification, and avoid the effects of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Synthesis of compound 2 (2α, 3α-epoxy-17-one-5α-androstane).
[0023] Add 5α-androst-2-en-17-one (27.2g, 0.1mol) and chloroform (140ml) into a clean and dry 250ml three-neck flask, stir to dissolve, cool down to 0-5°C, and control The internal temperature is lower than 5°C, slowly add m-CPBA (16.0g, 0.102mol) in chloroform solution (32ml), control the internal temperature at 0-5°C, stir for 5-6h, and use 1.5mol / L for the reaction solution Wash with ammonia water (60ml*2), then wash with water until neutral, and use starch potassium iodide test paper to detect m-CPBA until it is completely removed. Wash the organic phase with saturated saline (30ml), and then add 5g of anhydrous sodium sulfate to dry for 4-5h. Filtrate, concentrate under reduced pressure to obtain 25.7g of oil, recrystallize from methanol, and dry in vacuo to obtain 22.4g of the target compound 2 (2α,3α-epoxy-17-one-5α-androstane), yield: 77.7%.
[0024] 2. Synthesis of compound 3 (2β-(4-morpholinyl)...
Embodiment 2
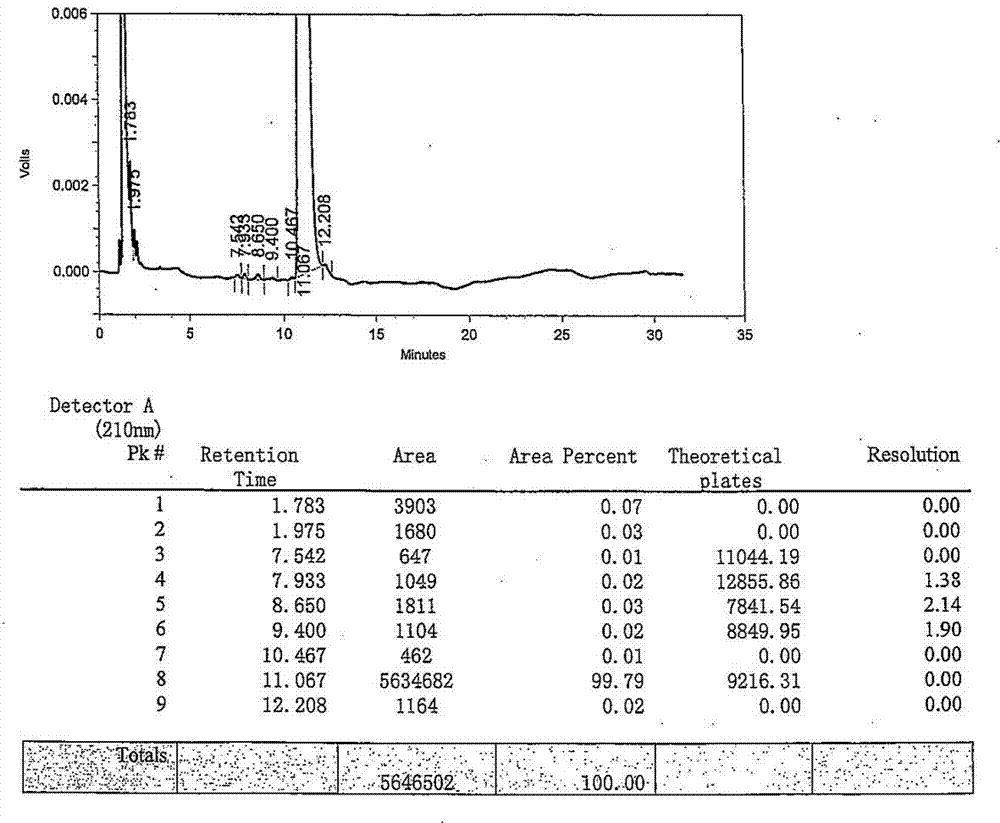
[0035] The product rocuronium bromide HPLC purity 99.79% of embodiment 1 gained, see figure 1 ;
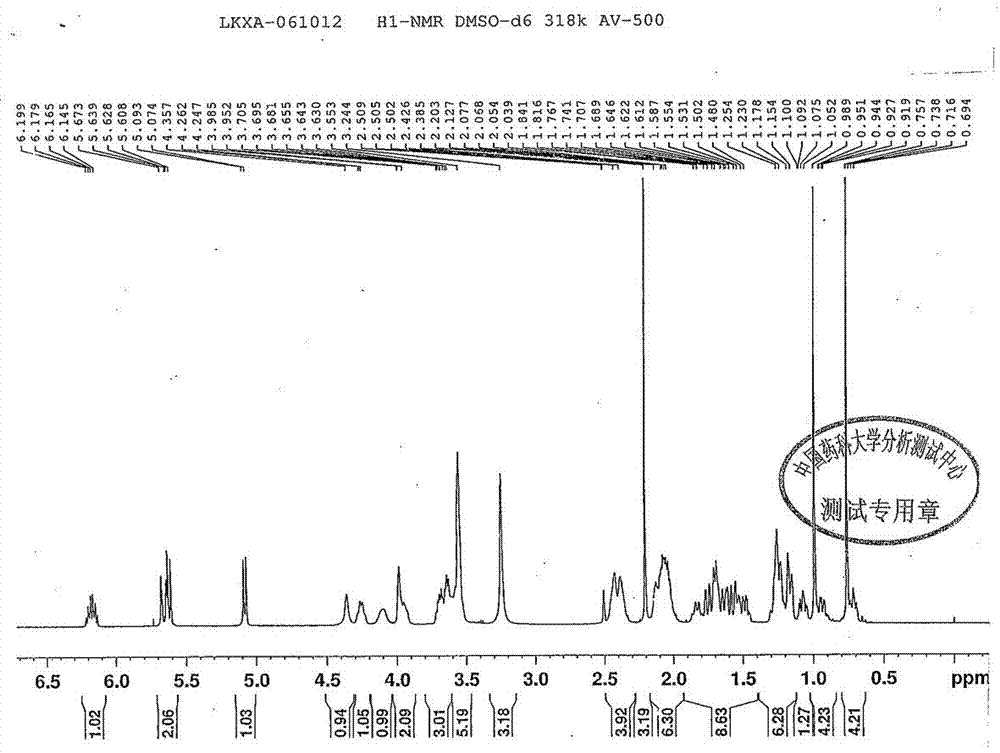
[0036] Structure confirmation data: mp: 162-166°C, H-NMR (500MHz, DMSO): δ0.694-0.797 (m, 4H), δ0.902-1.030 (m, 4H), δ1.052-1.092 (m, 1H), δ1.075-1.390 (m, 6H), δ1.400-1.925 (m, 9H), δ1.930-2.170 (m, 6H), δ2.203 (s, 3H), δ2.300-2.490 (d, 4H), δ3.244 (s, 3H), δ3.533 (s, 5H), δ3.610-3.740 (m, 3H), δ3.900-4.100 (m, 2H), δ4.098 ( s, 1H), δ4.247-4.262 (dd, 1H), δ4.357 (s, 1H), δ5.074-5.093 (d, 1H), δ5.608-5.673 (m, 2H), δ6.131 -6.213 (m, 1H), see figure 2 .
[0037] Compared with the prior art, the present invention has the following advantages:
[0038]1. Using compound 1 (5α-androst-2-en-17-one) as a raw material, the morpholine ring is introduced by first opening the epoxy ring on the six-membered ring, and then introducing a functionalized five-membered ring ketone at the α-position four Hydrogen pyrrole avoids the problem of chemoselectivity of tetrahydropyrrole opening epo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com