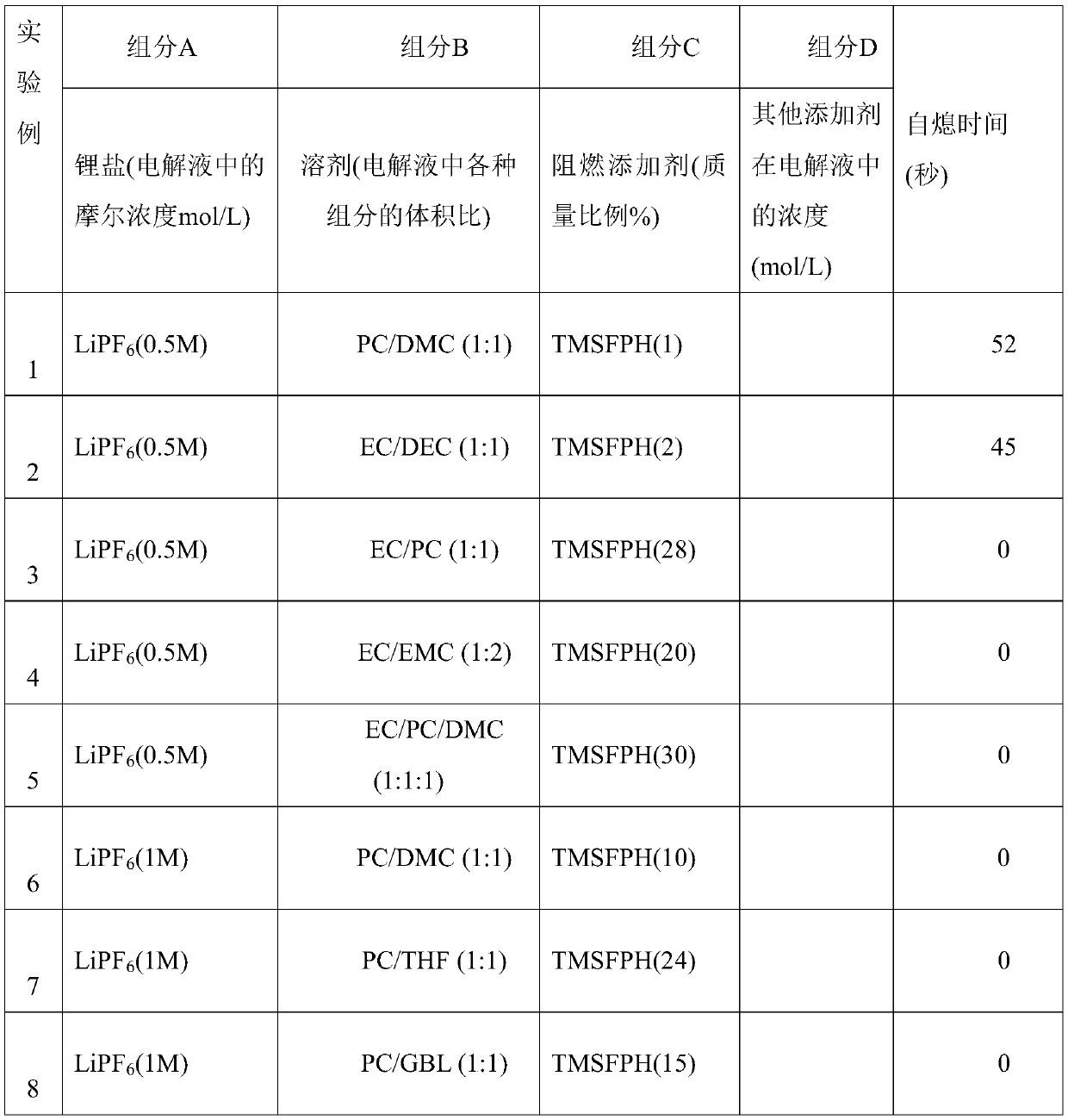
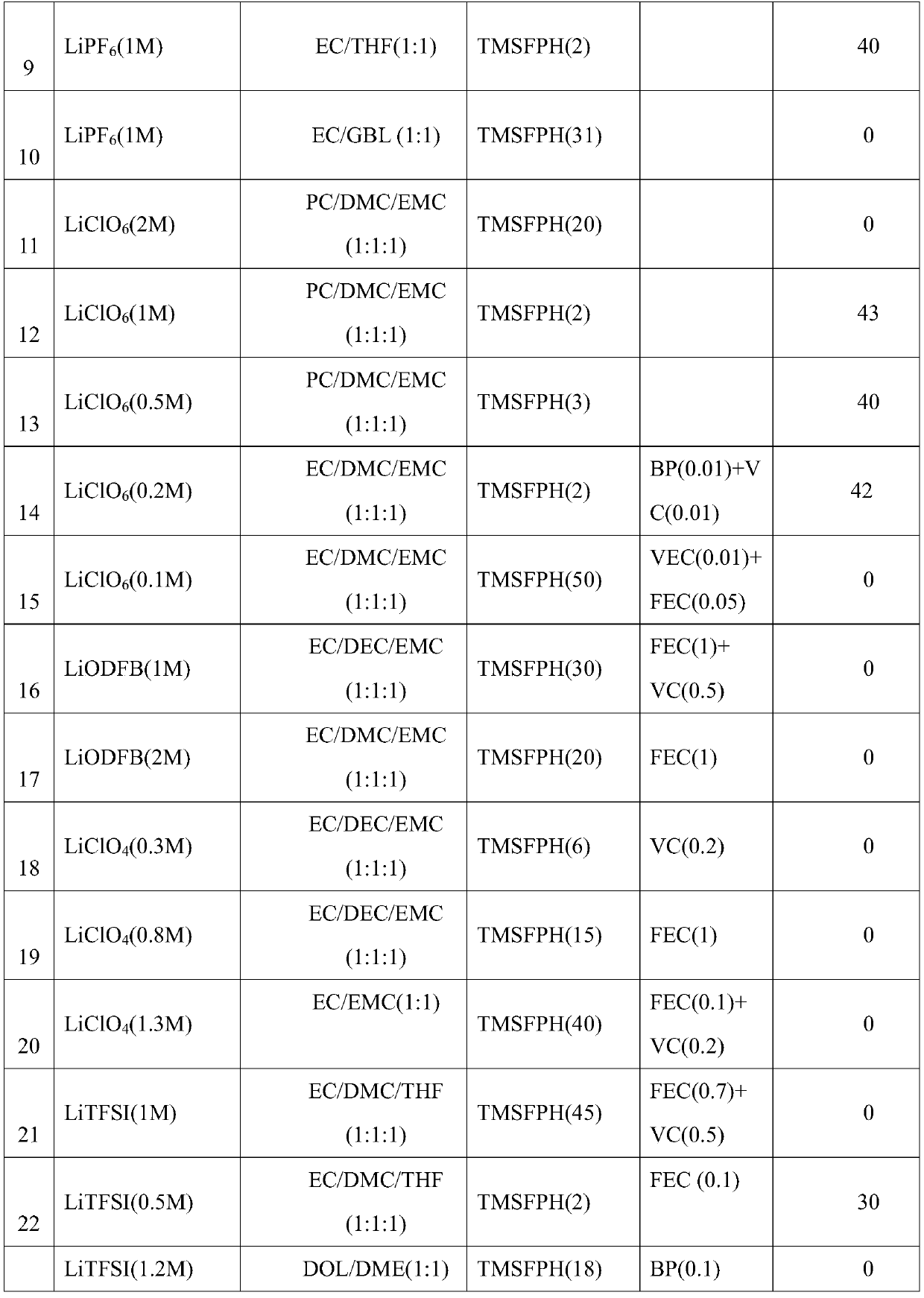
A kind of flame retardant siloxy fluorocyclotriphosphazene and its synthesis method
A technology of siloxy fluoro cyclotriphosphazene and fluoro cyclotriphosphazene, which is applied in the field of flame retardant siloxy fluoro cyclotriphosphazene and its synthesis, and can solve the problem that the fire and combustion of batteries cannot be completely prevented. Even explosion, can not significantly improve the flash point of lithium-ion battery electrolyte, negative impact on lithium battery performance and other problems, to achieve good thermal stability and radiation resistance and weather resistance, easy industrial production, good reaction controllability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a method for synthesizing siloxyfluorocyclotriphosphazene, and the prepared siloxyfluorocyclotriphosphazene is used in high-performance lithium-ion battery electrolyte additives.
[0028] In the siloxy fluorocyclotriphosphazene compound, the substitution of the siloxy group can be mono-substituted, partially substituted or fully substituted.
[0029] The present invention also provides a synthetic method of siloxyfluorocyclotriphosphazene, comprising the following steps:
[0030] Dissolving the fluorinated cyclotriphosphazene in an organic solvent, adding a siloxy compound, selecting a catalyst, reacting for 0.5-72 hours, replacing the fluorine atom with a siloxy group to obtain a siloxyfluorocyclotriphosphazene.
[0031] Preferably, the siloxy compound is potassium trimethylsiliconate, sodium trimethylsiliconate or lithium trimethylsiliconate. It has been verified by experiments that the siloxylation effect of these three siloxy compounds is bet...
Embodiment 1
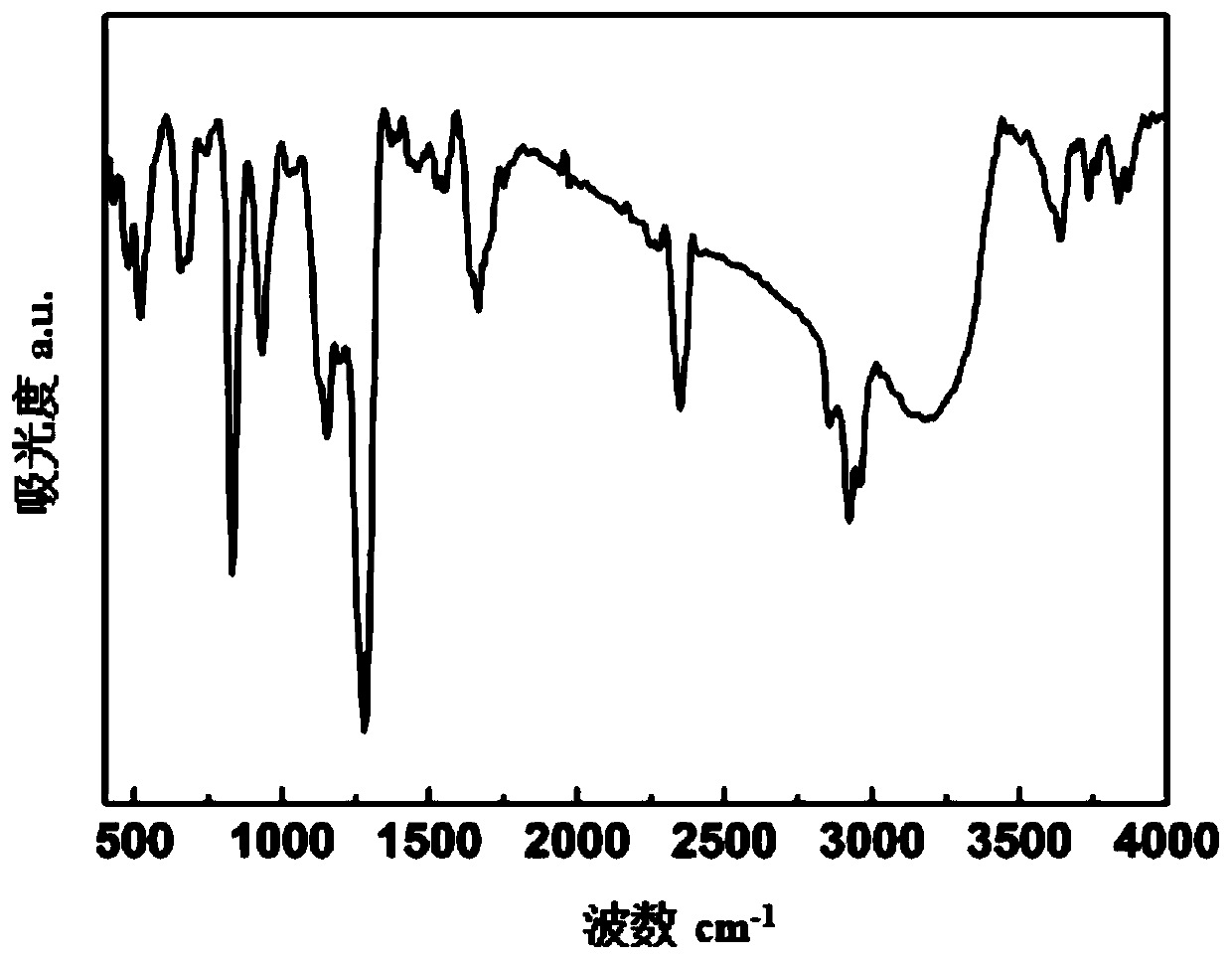
[0047] 49.8g of hexafluorocyclotriphosphazene crystals are dissolved in 200ml of tetrahydrofuran to form a hexafluorocyclotriphosphazene solution, and 12.9g of potassium trimethylsiliconate and a small amount of graphene are added to the solution as a catalyst, stirred until uniform, and in 10 React for 20 hours at ℃, filter and distill under reduced pressure to obtain the phosphazene derivative siloxyfluorocyclotriphosphazene, which has the following structural formula, with a yield of 95%, and its structure is determined by infrared, as shown in figure 1 shown.
Embodiment 2
[0049] 49.8g of hexafluorocyclotriphosphazene crystals were dissolved in 300ml of n-hexane to form a hexafluorocyclotriphosphazene solution, and 12.9g of potassium trimethylsiliconate and an appropriate amount of Raney nickel were added to the solution as a catalyst, stirred until uniform, and React at 40°C for 10 hours, filter and distill under reduced pressure to obtain the phosphazene derivative, siloxyfluorocyclotriphosphazene, with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com