Method for preparing porous and spherical basic magnesium carbonate crystal by utilizing magnesite
A magnesite and magnesium carbonate technology, applied in the direction of magnesium carbonate, can solve the problems of waste of resources and reduction of high-quality mineral resources, and achieve the effects of reducing preparation cost, reducing probability of occurrence and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
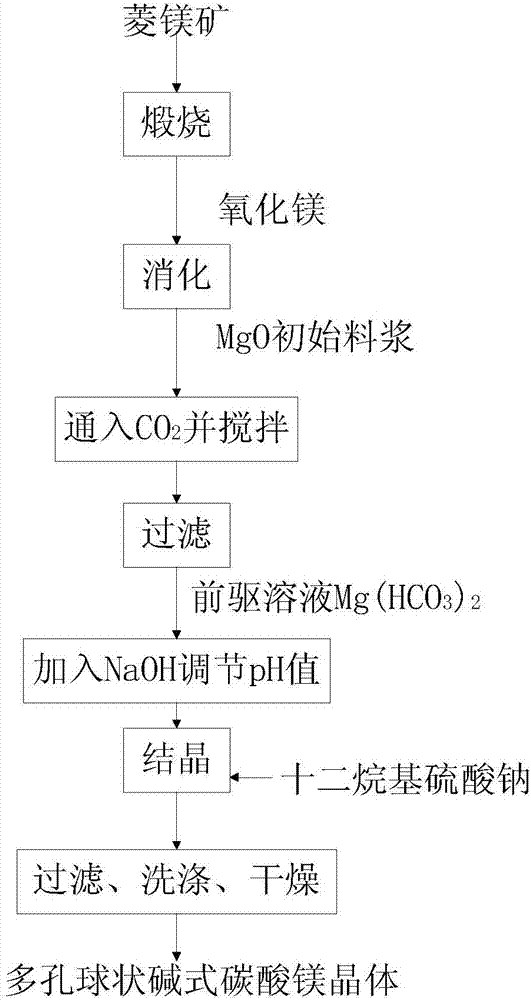
[0053] A kind of method that utilizes magnesite to prepare porous spherical basic magnesium carbonate crystal, its process flow diagram is shown in figure 1 , including the following steps:
[0054] (1) Calcined magnesite
[0055] The magnesite was calcined at 750°C for 3.0 hours, so that the decomposition rate of magnesite reached 99%, and active magnesium oxide was obtained. The activity was detected by the citric acid method, and the discoloration time was 30s;
[0056] Grinding the activated magnesium oxide to -74 μm to obtain activated magnesium oxide powder;
[0057] (2) Digest and prepare Mg(OH) 2 suspension
[0058] Using the obtained activated magnesia powder as raw material, add deionized water at 90°C, fully stir to prepare MgO initial slurry; wherein, according to the mass ratio, activated magnesia powder: deionized water = 1:40;
[0059] Put the initial slurry of MgO in a constant temperature water bath at 60°C, stir for 3.5 hours, and air-cool to room tempera...
Embodiment 2
[0069] A method utilizing magnesite to prepare porous spherical magnesium basic carbonate crystals, comprising the steps of:
[0070] (1) Calcined magnesite
[0071] The magnesite was calcined at 700°C for 3.0 hours, so that the decomposition rate of magnesite was 98%, and active magnesium oxide was obtained. The activity was detected by the citric acid method, and the discoloration time was 28s;
[0072] The activated magnesium oxide is pulverized into activated magnesium oxide powder with a particle size of -72 μm;
[0073] (2) Digest and prepare Mg(OH) 2 suspension
[0074] Using the obtained activated magnesia powder as raw material, add deionized water at 70°C, fully stir to prepare MgO initial slurry; wherein, according to the mass ratio, activated magnesia powder: deionized water = 1:50;
[0075] Put the initial MgO slurry in a constant temperature water bath at 55°C, stir for 5.0 h, and air cool to room temperature to obtain Mg(OH) 2 suspension;
[0076] (3) Prepara...
Embodiment 3
[0083] A method utilizing magnesite to prepare porous spherical magnesium basic carbonate crystals, comprising the steps of:
[0084] (1) Calcined magnesite
[0085] The magnesite was calcined at 800°C for 1.0h, so that the decomposition rate of magnesite was 99%, and active magnesium oxide was obtained. The activity was detected by the citric acid method, and the discoloration time was 30s;
[0086] Grinding activated magnesium oxide to activated magnesium oxide powder with a particle size of -70 μm;
[0087] (2) Digest and prepare Mg(OH) 2 suspension
[0088] Using the obtained activated magnesia powder as raw material, add deionized water at 100°C, fully stir to prepare MgO initial slurry; wherein, according to the mass ratio, activated magnesia powder: deionized water = 1:12;
[0089] Put the initial MgO slurry in a constant temperature water bath at 65°C, stir for 1.0 h, and air cool to room temperature to obtain Mg(OH) 2 suspension;
[0090] (3) Preparation of precu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com