A preparation method of modified cyclodextrin/carboxymethyl chitosan nanoparticles stably embedding anthocyanins
A technology of carboxymethyl chitosan and nanoparticles, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve problems such as poor stability and achieve benefits to the human body. Absorption, good stabilization, mild reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of modified cyclodextrin / carboxymethyl chitosan nanoparticles with stable embedded anthocyanins
[0035] (1) Dissolve 1.5 g of β-cyclodextrin (β-CD) in 5 mL of anhydrous dimethyl sulfoxide and 5 mL of isopropanol mixture, add 12.5 mL of 1M NaOH aqueous solution, and then add 2 mL Of epichlorohydrin. Stir under nitrogen for 48h at room temperature. The pH of the reaction solution was adjusted to 7.0 with hydrochloric acid, and the reaction mixture was precipitated with excess acetone. The reaction mixture precipitated by acetone was dissolved in a small amount of deionized water, transferred to a dialysis bag (MWCO, 1000 Da), and purified by dialysis using deionized water as the dialysis medium at room temperature. After the dialysis, the samples in the dialysis bag were collected and freeze-dried to obtain purified modified cyclodextrin (β-CD-EP).
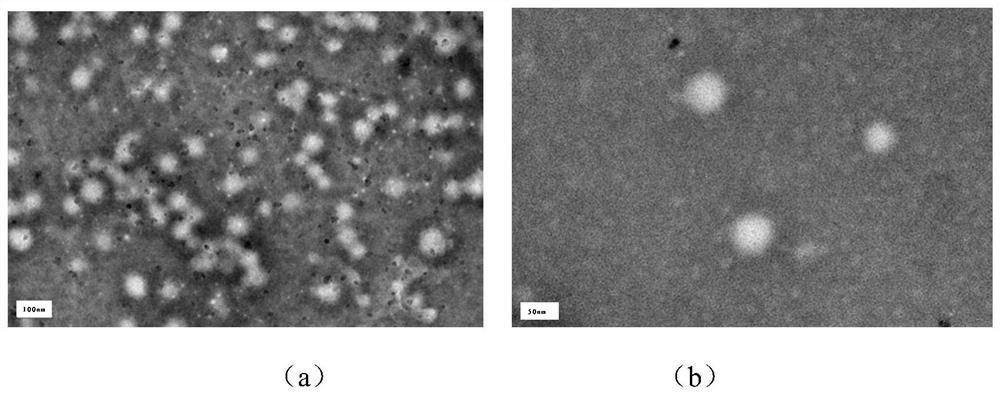
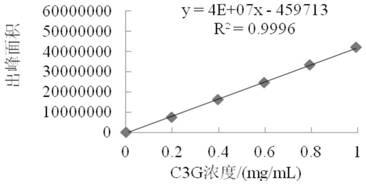
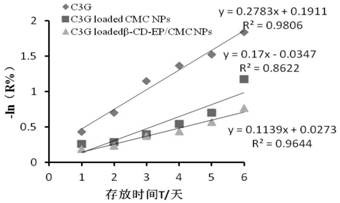
[0036] (2) Preparation of anthocyanin-loaded modified cyclodextrin carboxymethyl chitosan nanoparticl...
Embodiment 2
[0040] Example 2: Step (1) is the same as Example 1, except that the carboxymethyl chitosan solution and CaCl in step (2) 2 The volume ratio of the solution is 5:1.
Embodiment 3
[0041] Example 3: Step (1) is the same as Example 1, except that the carboxymethyl chitosan solution and CaCl in step (2) 2 The volume ratio of the solution is 5:3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com