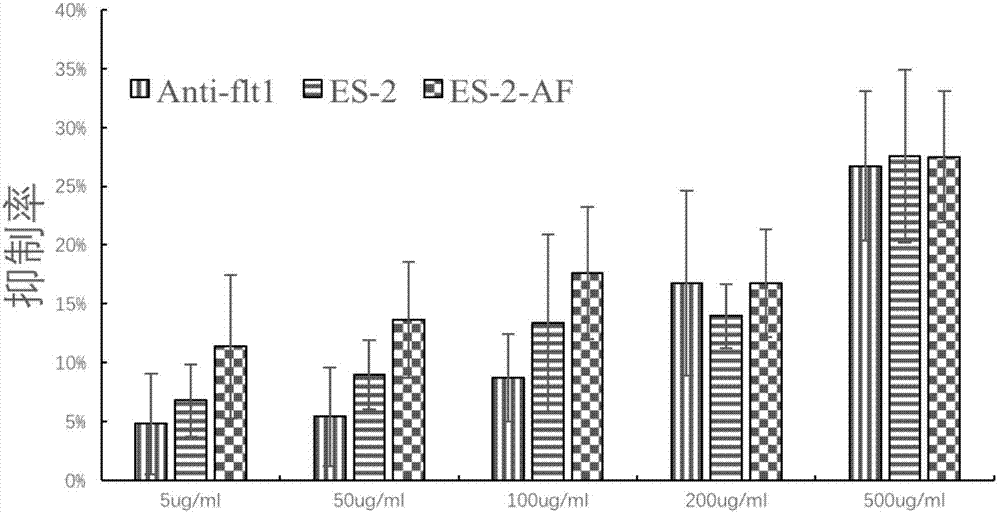
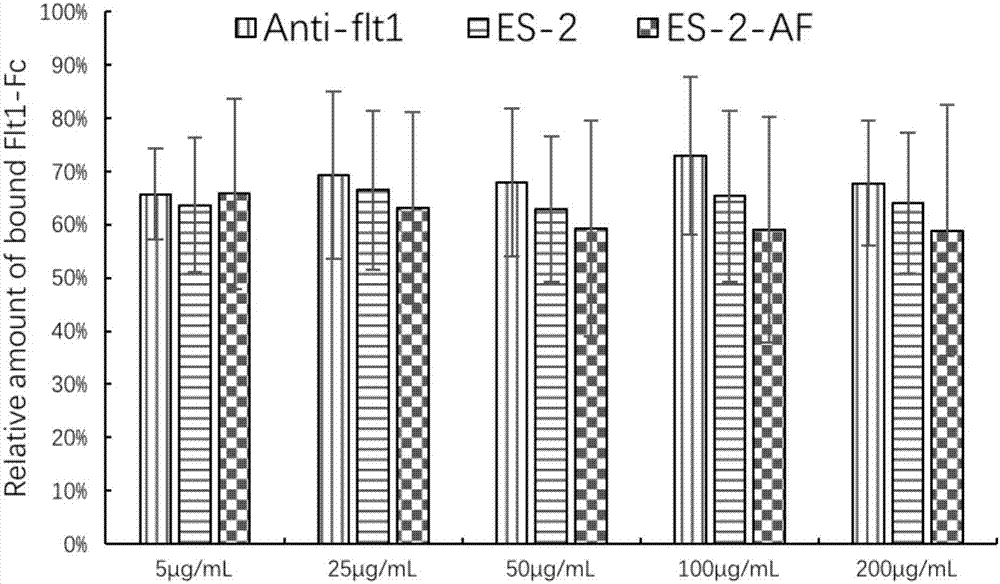
Angiogenesis inhibiting peptide, hyaluronic acid modified compound thereof and preparation method and application of hyaluronic acid modified compound
A technology of hyaluronate and modifier, applied in the field of biomedicine, can solve the problems of limited application of polypeptide drugs, poor water solubility, poor stability, etc., and achieve the effects of strong activity, increased half-life and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
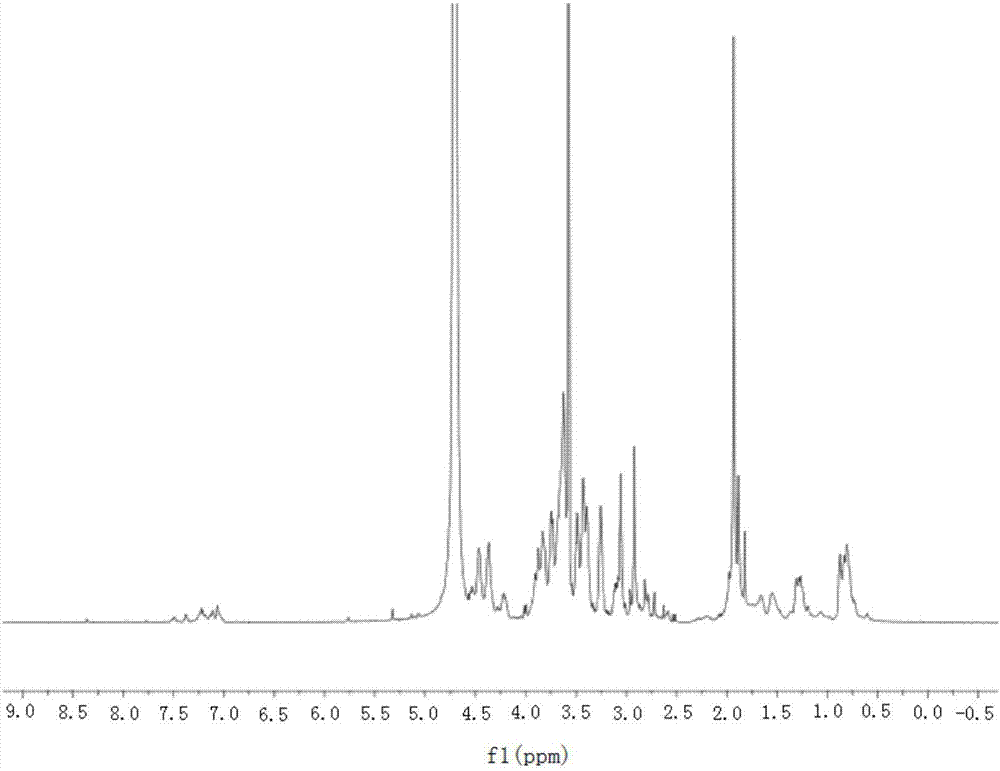
[0053] Embodiment 1: Preparation of ES-2-AF peptide
[0054] The molecular weight of the ES-2-AF peptide of the present invention is 2197.5Da. It is synthesized by Fmoc solid phase, purified by high performance liquid phase, and confirmed by means of mass spectrometry and high performance liquid chromatography. The purity can reach more than 95%. .
Embodiment 2
[0055] Example 2: Preparation of ES-2-AF peptide hyaluronic acid modification (HA-ES-2-AF)
[0056] The preparation steps are as follows:
[0057] (1) Wash the Dowex ion exchange resin with water, add excess tetrabutylamine hydroxide TBA-OH (24.5ml), and mix well. Dowex-TBA resin was obtained, and the supernatant was removed by filtration. Sodium hyaluronate NaHA (1 g) was dissolved in water and poured into the prepared Dowex-TBA resin (10 g). After mixing for 3 hours, filter with a 0.45 μm syringe filter to remove Dowex resin to obtain a clear HA-TBA solution, which was lyophilized for 3 days.
[0058] (2) HA-TBA and ES-2-AF peptide prepared in step (1) were dissolved in DMSO respectively. After HA-TBA is fully dissolved, add excess Carter condensing agent BOP to activate HA carboxyl, and mix for 30 minutes. Then the HA-TBA solution, the peptide solution, and an equimolar amount of DIPEA were mixed and dissolved in DMSO. After reacting for 24 hours, an equal volume of 1M ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com