Method for preparing sofosbuvir intermediate and recovering byproduct
A by-product, asana technology, applied in the field of medicine, can solve the problems of harsh reaction conditions, emulsification of the system, affecting the yield, etc., and achieve the effects of easy industrial production, mild reaction conditions, and easy to enlarge the output.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 300mL ethylene glycol dimethyl ether, 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribonic acid-γ-lactone (50g, 134mmol ), cooled to 10°C, and added sodium borohydride (2.3g, 60mmol, 0.45eq) in three batches, with an interval of one hour between each batch. After the addition, the temperature was naturally raised to room temperature, and the reaction was stirred for 2 hours, followed by spot plate analysis by TLC. The disappearance of raw material spots in the reaction solution was the end of the reaction. After the reaction was completed, the pH was adjusted to 6-7 with acetic acid, and solids were precipitated, and the stirring was continued for 12 hours.
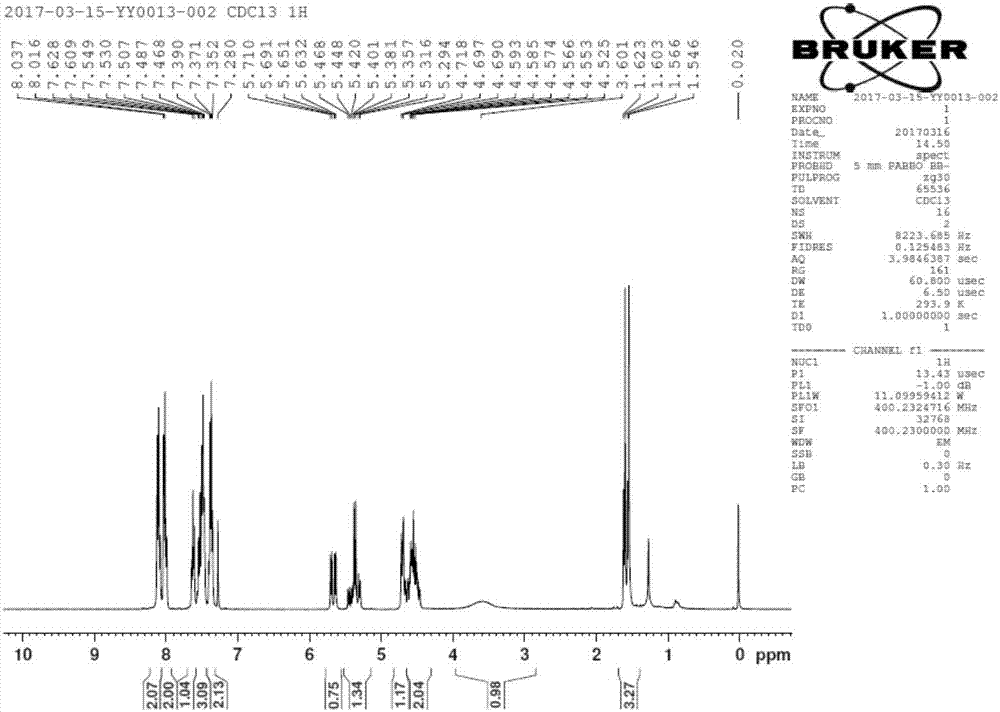
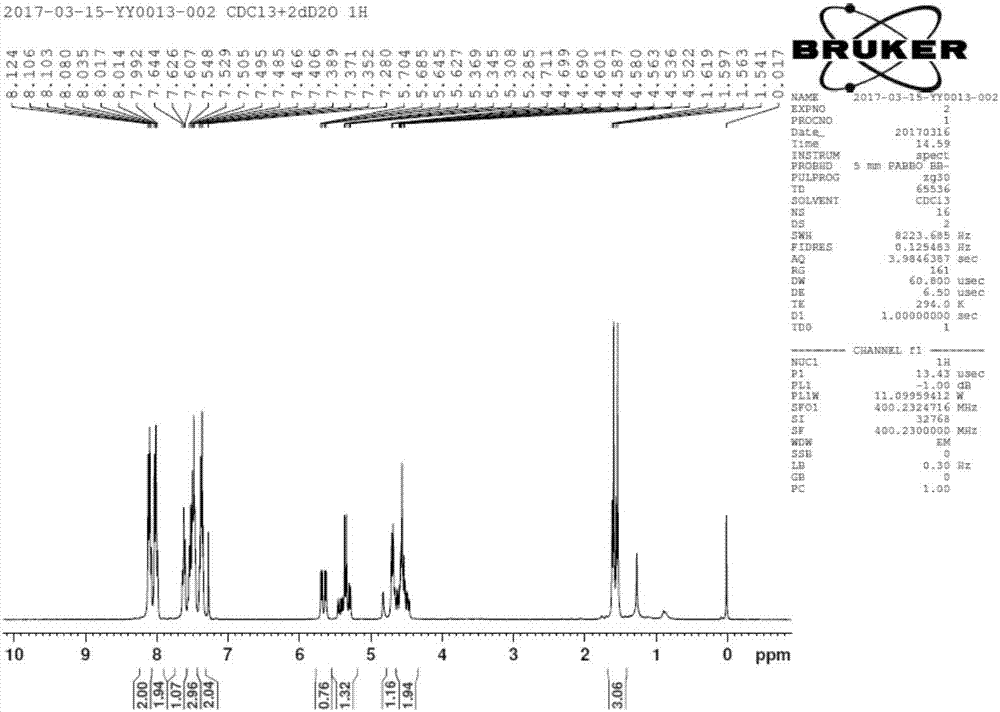
[0043] Filtration; drying the obtained filter cake to obtain the by-product [(2R,3R,4S)-4-fluoro-2,5-dihydroxy-4-methylpentane-1,3-diyl]diphenyl Ester 23.7g, 63mmol. ( 1 H NMR (400MHz, CDCl 3 )δ8.05-7.99(m,4H),7.61-7.37(m,6H),5.55(dd,J=18.0,6.4Hz,1H),4.72-4.37(m,3H),3.89-3.82(m, 2H), 3.64 (s, 2H, -OH), 1.46 (...
Embodiment 2
[0056] Add 300mL ethylene glycol dimethyl ether, 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribonic acid-γ-lactone (50g, 134mmol) into a 500mL reaction bottle , cooled to 10° C., and sodium borohydride (1.3 g, 34 mmol, 0.25 eq) was added in three batches with an interval of one hour between each batch. After the addition was completed, the temperature was naturally raised to room temperature, and the reaction was stirred for 10 hours. The spot plate analysis was carried out by TLC, and the raw materials were slightly left. The pH was adjusted to 6-7 with acetic acid, a solid precipitated out, and the stirring was continued for 12 hours. Filter and dry the filter cake to obtain the by-product [(2R,3R,4S)-4-fluoro-2,5-dihydroxy-4-methylpentane-1,3-diyl]dibenzoate 20.3 g, 54 mmol. The filtrate was concentrated to dryness on a rotary evaporator under reduced pressure, dissolved in 400 mL of ethyl acetate, washed with 150 mL of saturated brine, the organic layer was dried over anh...
Embodiment 3
[0060] Add 300mL ethylene glycol dimethyl ether, 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribonic acid-γ-lactone (50g, 134mmol) into a 500mL reaction bottle , cooled to 10° C., and sodium borohydride (5.1 g, 134 mmol, 1.0 eq) was added in three batches with an interval of one hour between each batch. After the addition, the temperature was naturally raised to room temperature, and the reaction was stirred for 1 hour, followed by spot plate analysis by TLC. The disappearance of raw material spots in the reaction liquid was the end of the reaction. After the reaction was completed, the pH was adjusted to 6-7 with acetic acid, and solids were precipitated, and the stirring was continued for 12 hours. Filter and dry the filter cake to obtain the by-product [(2R,3R,4S)-4-fluoro-2,5-dihydroxy-4-methylpentane-1,3-diyl]dibenzoate 35.3 g, 94 mmol. The filtrate was concentrated to dryness on a rotary evaporator under reduced pressure, dissolved in 400 mL of ethyl acetate, washed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com