Engineering mechanically functional human cartilage and method of making same
An engineered, human technology used in pharmaceutical formulations, prostheses, joint implants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0067] Bone marrow-derived human mesenchymal stem cells were obtained from Cambrex with fresh human bone marrow aspirates. Bone marrow-derived human mesenchymal stem cells (hMSCs) were isolated by attaching them to a plastic surface. Cells were expanded in DMEM supplemented with 10% FBS, 1% pen-strep, and 0.1 ng / mL basic fibroblast growth factor. hMSCs were cultured to the third passage and shown to exhibit multi-lineage differentiation capacity.
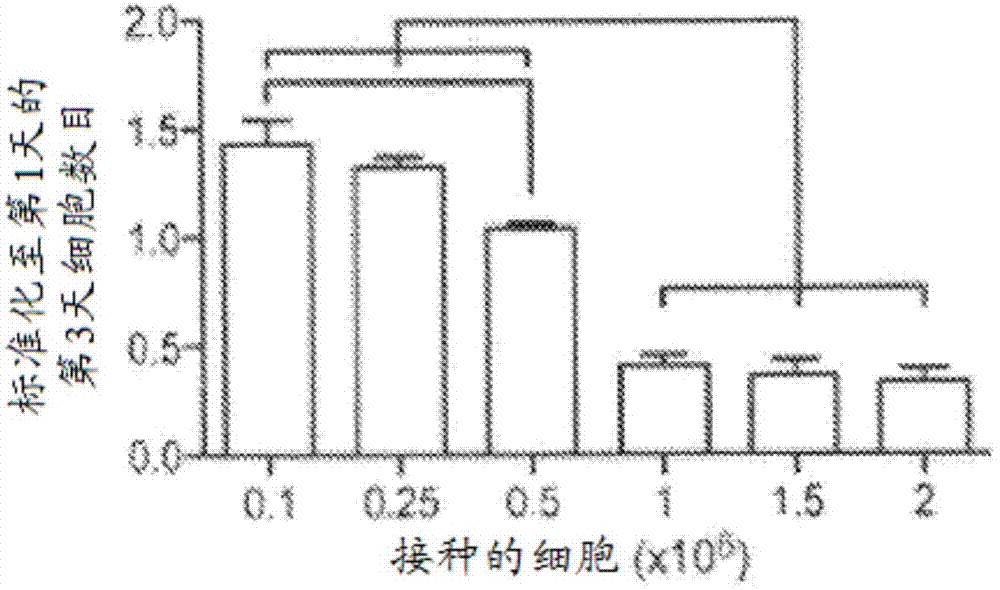
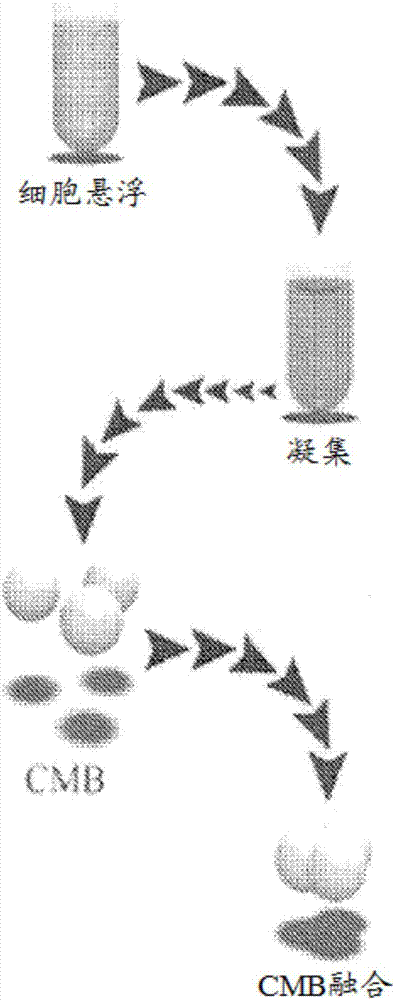
[0068] Generation of condensed mesenchymal bodies (CMBs) hMSCs were suspended in culture medium (supplemented with 100 nM dexamethasone; 50 μg / ml ascorbate-2-phosphate; 100 μg / ml sodium pyruvate; 40 μg / ml proline ; 1% insulin, transferrin, sodium selenite [ITS+] mixture; 1% antibiotic and 10 ng / ml transforming growth factor-β3 [TGF-β3] in high glucose DMEM). To determine the optimal size of CMBs, hMSCs were divided into 10 5 , 2.5×10 5 , 5×10 5 , 106, 1.5×10 6 and 2×10 6 cells / ml concentration. Add 1ml of cell suspension to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Young's modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com