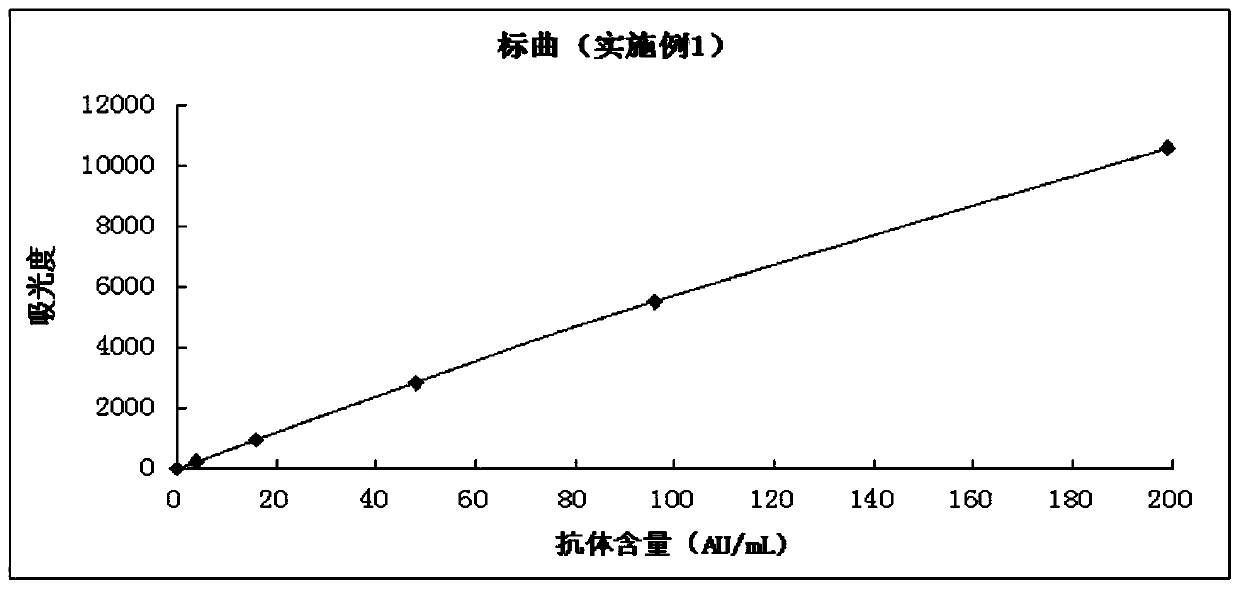
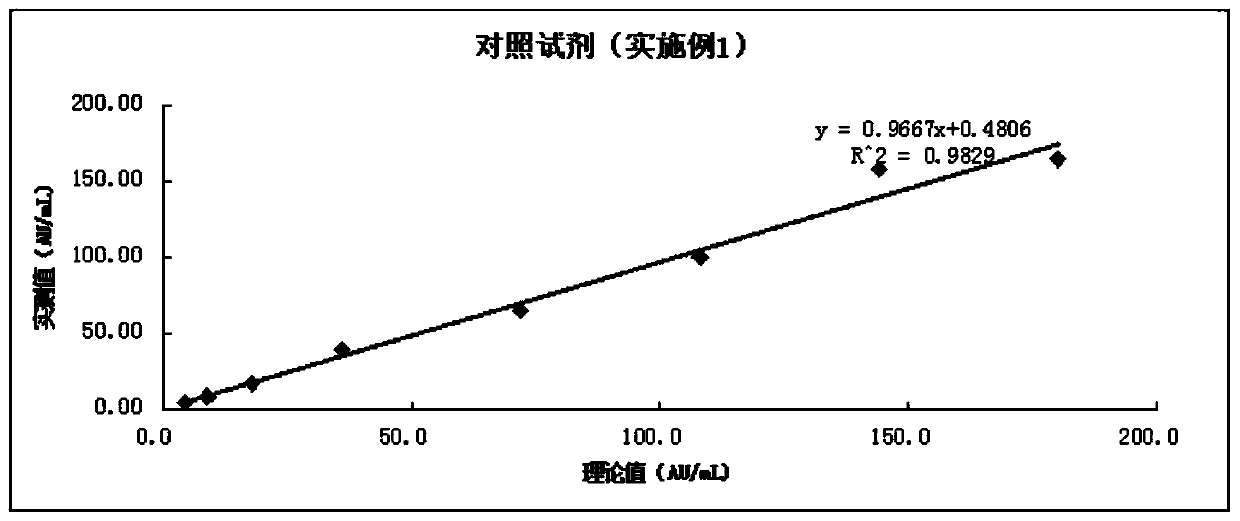
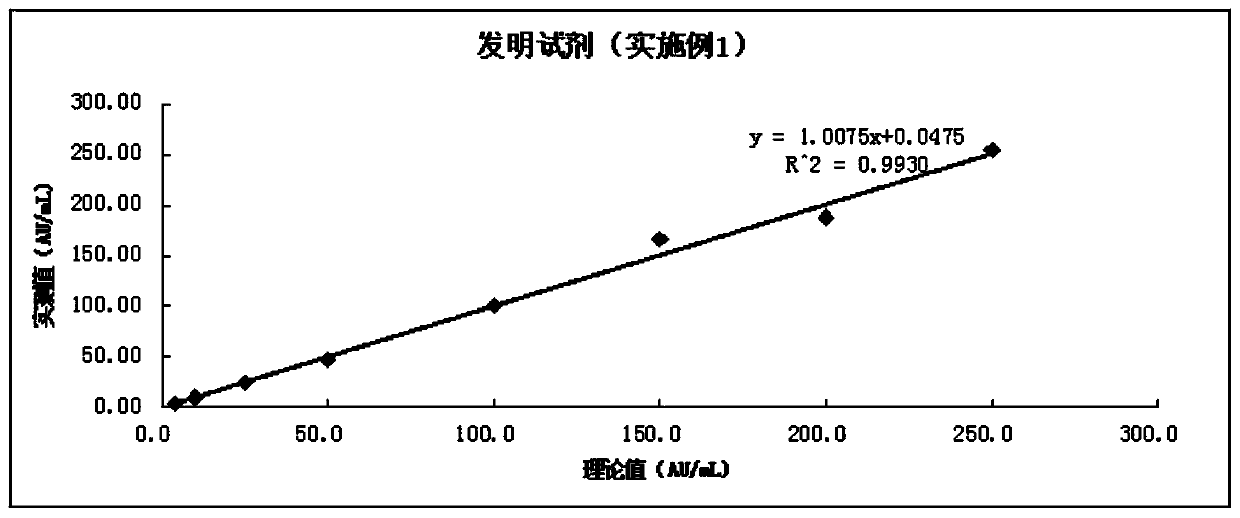
A modified latex immunoturbidimetric assay kit for improving the detection rate of antibodies against Helicobacter pylori pathogenic strains
A technology of Helicobacter pylori and pathogenic bacteria, applied in the field of medical testing, can solve the problems of low specificity and accuracy, affecting sensitivity, stability of cross-linking agents and latex microspheres, specificity and coupling efficiency, etc. , to achieve the effect of increasing linearity and sensitivity, improving sensitivity and shortening activation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Preparation of antibody assay kit for Helicobacter pylori pathogenic strains
[0053] Preparation of Helicobacter pylori Antigen in East Asia
[0054] 1) Isolation, cultivation and identification of gastric Helicobacter pylori: The gastric mucosal tissue of patients infected with Helicobacter pylori was bitten and examined through a gastroscope, and then the gastric mucosal tissue was ground into a slurry in a glass grinder containing 0.5 mL of Brooke's broth, and absorbed Spread 100 μL on the Columbia selection medium plate (containing 8% defibrinated sheep blood, 5mg / LTMP, 5mg / L polymyxin B, 5mg / L amphotericin B, 10mg / L vancomycin) , and then place the plate in a three-gas incubator (10% CO 2 , 85%N 2 , 5%O 2 ) at 37°C for 3 to 7 days, and carry out Gram staining microscopic examination, urease, catalase, and oxidase tests on the suspicious colonies cultured. The results are Gram-negative, S-type or arc-type. It was initially identified as Helicobacter pylori (...
Embodiment 2
[0129] 1. Preparation of antibody assay kit for Helicobacter pylori pathogenic strains
[0130] The preparation of East Asian Helicobacter pylori antigen is the same as that in Example 1 "Preparation of East Asian Helicobacter pylori antigen".
[0131] Preparation of reagent 1: 50mmol / L Tris-HCl buffer, add 0.8% sodium chloride, 0.5% BSA, 0.1% TRITON X-100, 5% sucrose, 0.05% dodecyl glucoside, 0.05% Proclin300 , each raw material should be stirred evenly after the addition of the raw materials, until the raw materials are added, continue to stir for 5 minutes, stand still for 2 minutes, adjust the pH to 8.0, and obtain reagent 1.
[0132] Preparation of Reagent 2:
[0133] 1) Modification of latex microspheres: dissolving hyperbranched polyglycidyl ether in pyridine, and modifying with succinic anhydride (Suc) to obtain hyperbranched polyglycidyl ether containing a large number of carboxyl groups.
[0134] 2) Latex particle labeling: Dilute the latex particle to 5mg / mL with ...
Embodiment 3
[0186] 1. Preparation of antibody assay kit for Helicobacter pylori pathogenic strains
[0187] The preparation of East Asian Helicobacter pylori antigen is the same as that in Example 1 "Preparation of East Asian Helicobacter pylori antigen".
[0188]Preparation of reagent 1: 50mmol / L Tris-HCl buffer, add 0.8% sodium chloride, 0.8% BSA, 0.5% TRITON X-100, 2% sucrose, 0.01% dodecyl glucoside, 0.05% Proclin300 , each raw material should be stirred evenly after the addition of the raw materials, until the raw materials are added, continue to stir for 5 minutes, stand still for 2 minutes, adjust the pH to 8.4, and obtain reagent 1.
[0189] Preparation of Reagent 2:
[0190] 1) Modification of latex microspheres: dissolving hyperbranched polyglycidyl ether in pyridine, and modifying with succinic anhydride (Suc) to obtain hyperbranched polyglycidyl ether containing a large number of carboxyl groups.
[0191] 2) Latex particle labeling: Dilute the latex particle to 5mg / mL with 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com