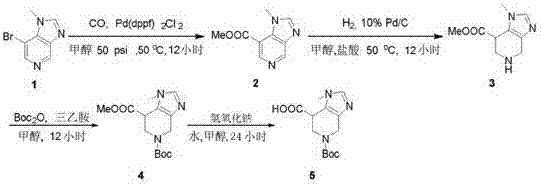
Synthetic method of 5-(boc t-butoxycarbonyl)-1-methyl-imidazopyridine-7-carboxylic acid
A technology of tert-butylcarbonyl and synthetic method, which is applied in the field of synthesis of 5-(tert-butylcarbonyl)-1-methyl-imidazopyridine-7-carboxylic acid, can solve problems such as no suitable industrial synthesis method, To achieve the effect of reasonable reaction process design, easy reaction and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0012] In the first step, compound 1 (50.0 g, 235.7 mmol) was dissolved in methanol (800 mL), triethylamine (180 mL) and Pd(dppf) were added 2 Cl 2 (8.00 g), stirred at 50 degrees Celsius for 12 hours under carbon monoxide pressure of 50 psi, added H after removing the solvent under reduced pressure 2 O ( 200 mL ) was dissolved, extracted with ethyl acetate (500 mL x 4), the combined organic phases were dried over anhydrous sodium sulfate, and the filtrate was concentrated to obtain compound 2 (73.00 g) as a yellow oil, and the crude product was directly used in the next step.
[0013] In the second step, compound 2 ( 19.00 g, 99.38 mmol ) was dissolved in methanol (500 mL), concentrated HCl ( 40 mL) and Pd / C (10.00 g) were added, and the reaction was carried out at 50°C for 12 hours under a hydrogen pressure of 50 psi. LC -MS monitored the reaction to be complete, filtered, and concentrated the filtrate to obtain compound 3 (73.00 g) as a yellow solid.
[0014] Step 3: Dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com