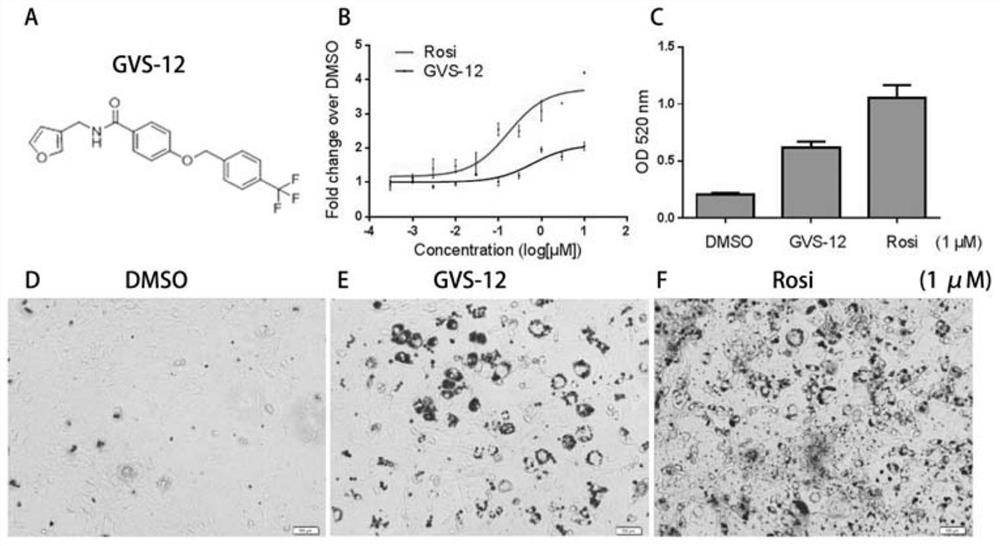
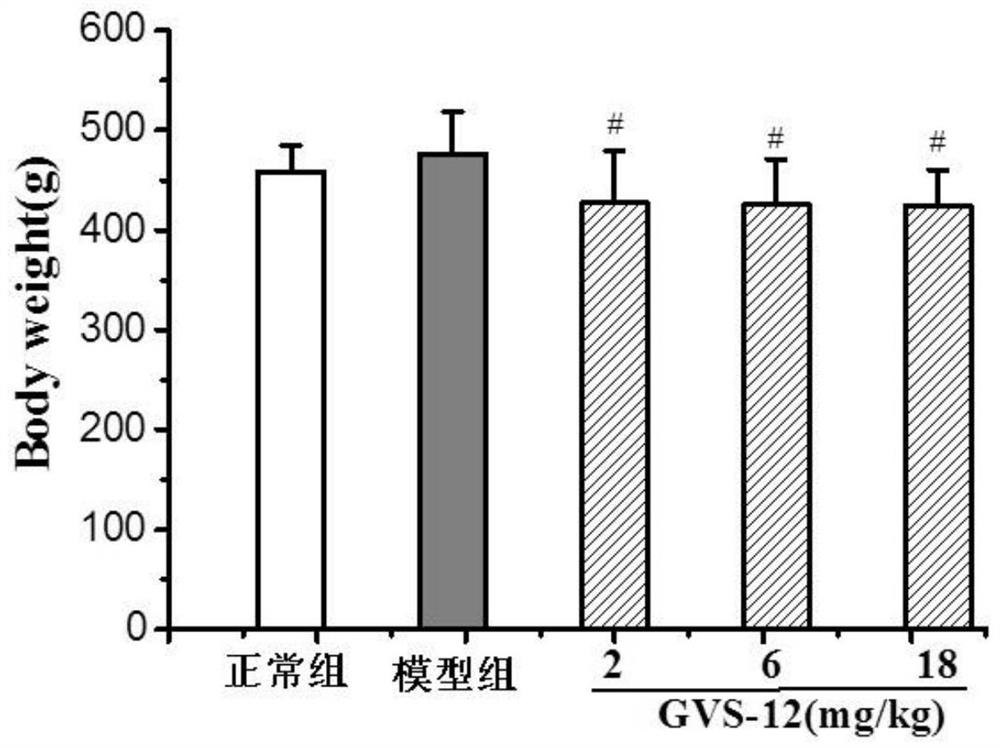
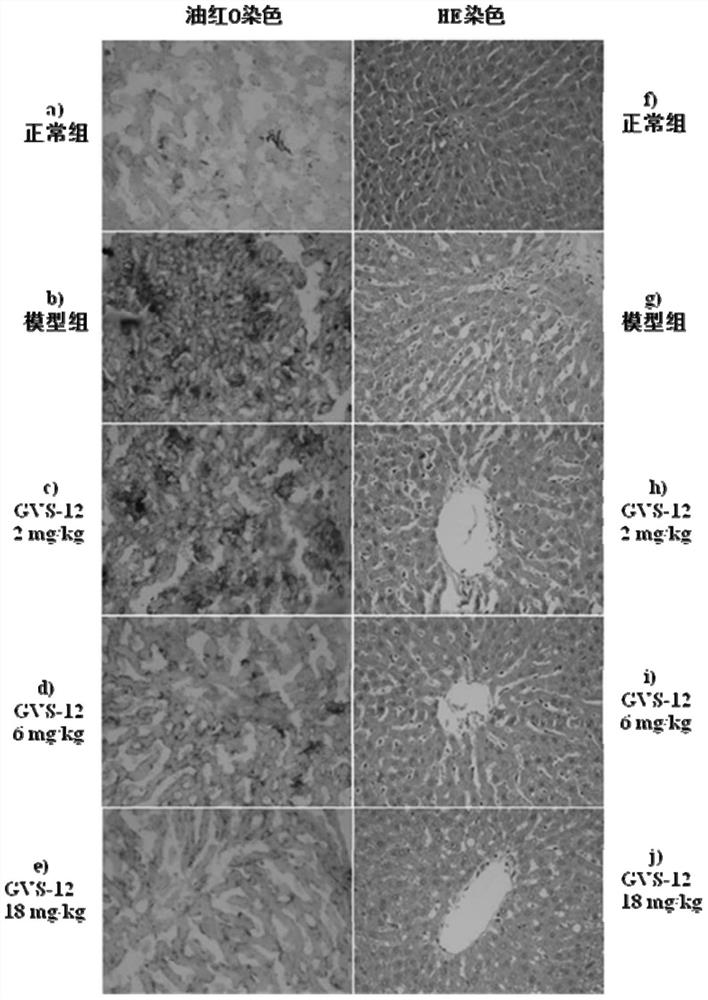
Novel gvs series compound and its application
A technology of compounds and uses, applied in the field of pharmaceutical applications, can solve the problems of patient intolerance, retention, weight gain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: Synthesis of GVS-1:
[0148]
[0149] Step 1: p-hydroxybenzoic acid (1.38g, 10mmol) and benzylamine (1.08g, 10mmol) were placed in a 25mL round bottom flask, BOP condensing agent (5.3g, 12mmol) and DMAP (1.46g, 12mmol) were added, Then add dry DMF 20mL, stir at room temperature, follow the reaction by TLC, after 3h the reaction is complete, EA extraction, washing and concentration to obtain intermediate 1, MS(EI): m / z 227.1 [M+H] + .
[0150] Step 2: Intermediate 1 (230 mg, 1 mmol) and 3,4-difluorobenzyl bromide (206 mg, 1 mmol) were placed in a 25 mL round bottom flask, Cs was added 2 CO 3 (490 mg, 1.5 mmol) in a flask, then add 8 mL of dry DMF, stir at room temperature, follow the reaction by TLC, the reaction is complete after 3 h, extract with water / ethyl acetate, until the aqueous phase has no compound, the combined organic phases are washed twice with water , washed with saturated NaCl solution, dried over anhydrous sodium sulfate, and finally sep...
Embodiment 2
[0151] Example 2: Synthesis of GVS-2:
[0152]
[0153] GVS-2 (85% yield) was prepared as GVS-1 using 3-fluoro-4-chlorobenzyl bromide instead of 3,4-difluorobenzyl bromide. 1 H NMR (400MHz, chloroform-d) δ 7.80–7.73 (m, 2H), 7.36 (d, J=4.4Hz, 4H), 7.33–7.25 (m, 2H), 7.25–7.10 (m, 2H), 7.01–6.93(m, 2H), 6.32(t, J=5.4Hz, 1H), 5.05(s, 2H), 4.64(d, J=5.6Hz, 2H). LCMS(ESI): m / z 354.1[ M+H] + .
Embodiment 3
[0154] Example 3: Synthesis of GVS-3:
[0155]
[0156] GVS-3 (81% yield) was prepared as GVS-1 using 4-trifluoromethylbenzyl bromide instead of 3,4-difluorobenzyl bromide. 1 H NMR (400MHz, chloroform-d) δ 7.80–7.74 (m, 2H), 7.69–7.61 (m, 2H), 7.54 (m, 2H), 7.35 (d, J=4.6Hz, 4H), 7.33– 7.27(m, 1H), 7.02–6.95(m, 2H), 6.34(s, 1H), 5.17(s, 2H), 4.63(d, J=5.6Hz, 2H). LCMS(ESI): m / z 386.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com