A recyclable magnetic chitosan phthalocyanine catalyst and its application
A chitosan and catalyst technology, applied in the field of magnetic chitosan phthalocyanine catalyst and its application, can solve the problems of complex synthesis process, use of additional light source, difficult recycling, etc., achieve simple synthesis steps, reduce the chroma of waste water, The effect of stabilizing physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
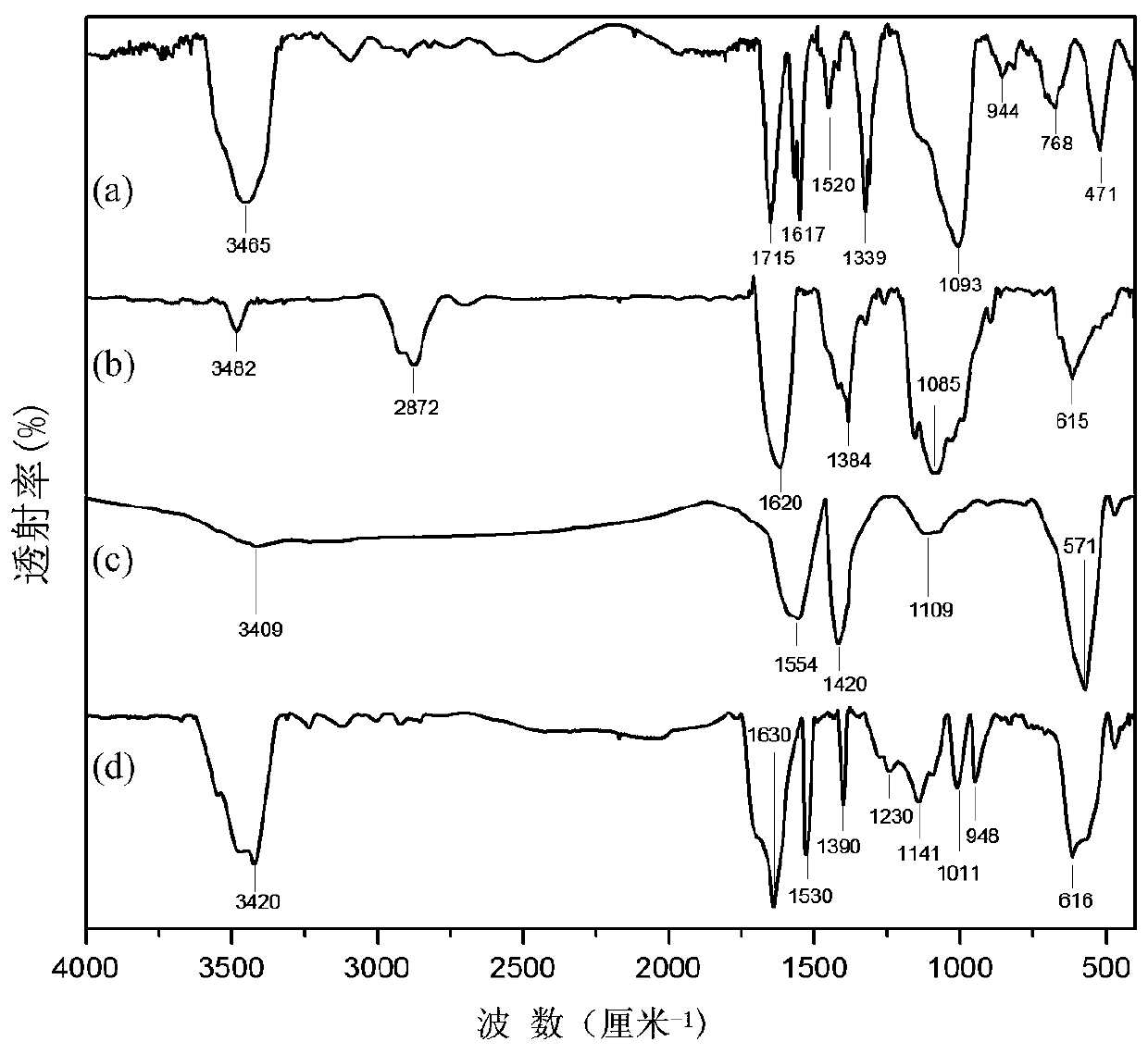
[0038] Step 1. Weigh 7.4 g of trimellitic anhydride, 1.363 g of cobaltous chloride, 15.015 g of urea, 0.148 g of ammonium molybdate, and 1.15 g of ammonium chloride into a three-necked flask, heat up to 120 °C and reflux for 2 hours, then heat up to 180 °C Keep warm for 6 hours.
[0039] Step 2. After cooling to room temperature, soak the obtained solid with 1mol / L hydrochloric acid for 5 hours, then pour the solution into water, filter, wash the filtered solid with deionized water 2 to 3 times, dry and collect.
[0040] Step 3. Weigh 5 g of the sample collected in step 2 and dissolve it in 200 ml of 2 mol / L sodium hydroxide saturated sodium chloride solution. After the solution is completely dissolved, heat the solution to 98 °C and heat under reflux for 8 hours.
[0041] Step 4. Suction filter the hydrolyzed reaction solution, add hydrochloric acid to the filtrate to adjust the pH to 2, and let stand to separate the obtained precipitate. The precipitate was washed with wate...
Embodiment 2
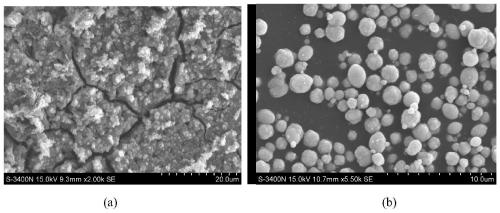
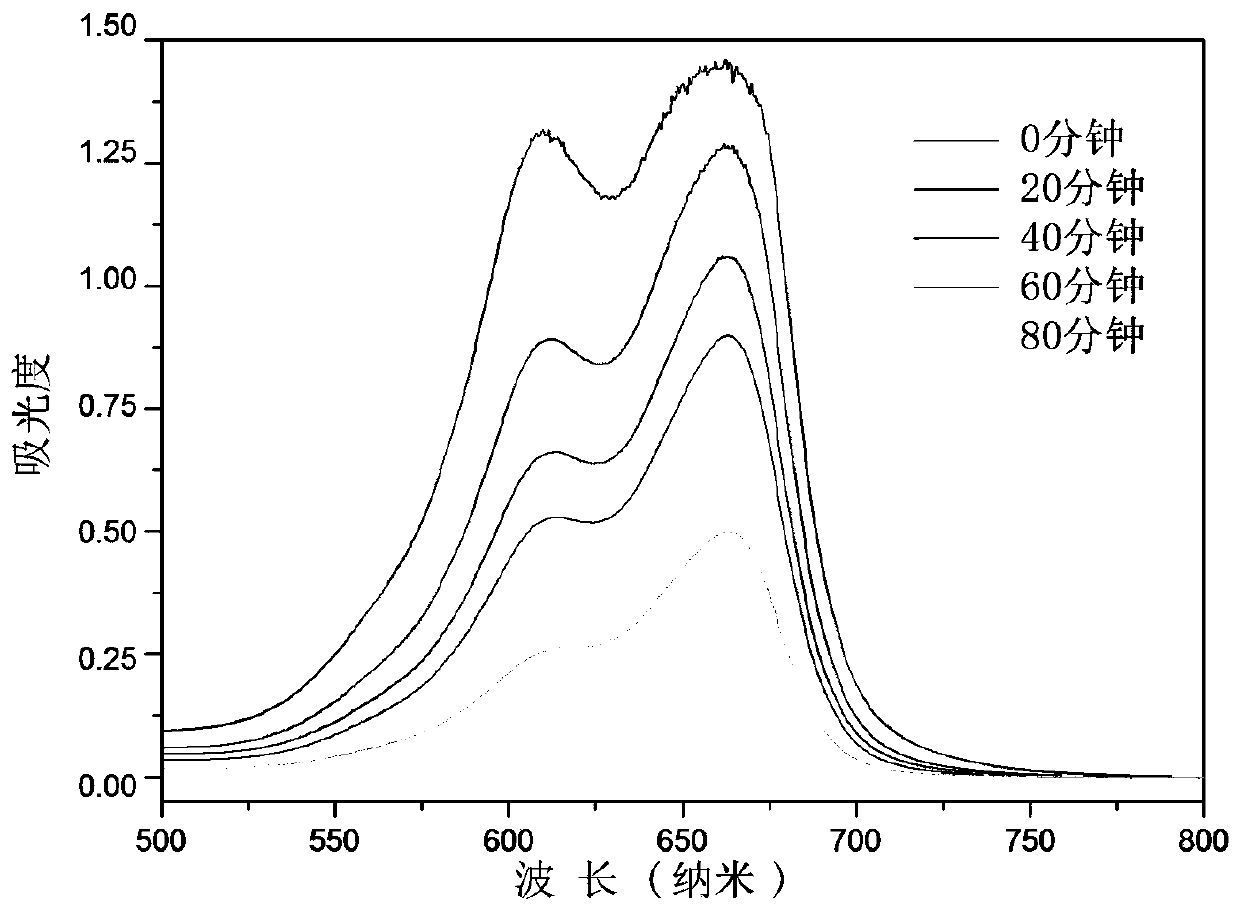
[0045] Add 0.1 g tetracarboxyphthalocyanine cobalt-chitosan magnetic powder into 200 mL of 50 ppm methylene blue solution, and stir at room temperature in the dark to make the catalyst and solution reach adsorption equilibrium. Afterwards, air was blown into the solution, and the stirring was continued under the irradiation of a fluorescent lamp. Measure the absorbance change of the solution at the maximum absorption wavelength (at 662nm), and calculate the degradation efficiency. The UV-Vis absorption spectrum changes within 1 hour of solution degradation as image 3 Shown, when reacting 4 hours, the absorbance of methylene blue in the solution reduces to original 10%, and dyestuff degradation rate reaches 90%. Reacted for 6 hours, the degradation rate of methylene blue reached 99.8%, and the degradation rate of methylene blue changed with time as shown in the attached Figure 4 shown. The magnetic catalyst was sucked out and separated by an external magnetic field, and th...
Embodiment 3
[0047] Add 10 mL of H to 200 mL of 50 ppm methylene blue solution dropwise 2 o 2 , Stir evenly under the condition of avoiding light, then add HCl to adjust the pH to 2, and then add 0.05g of tetracarboxyphthalocyanine cobalt-chitosan magnetic powder. Stirring under the condition of avoiding light and introducing air, it was found that the degradation rate of methylene blue reached 98.6% in 5 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com