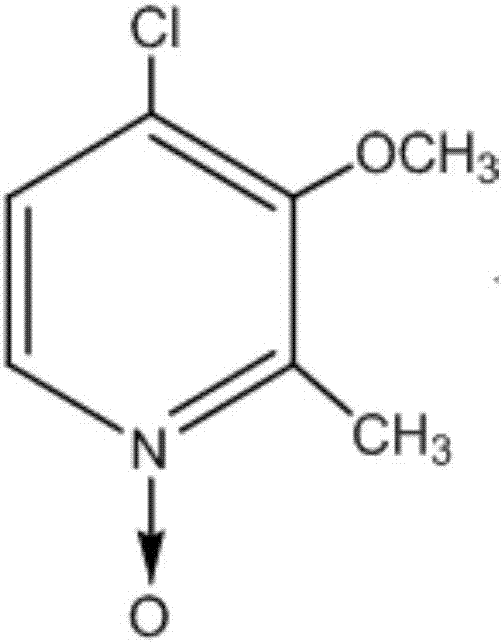
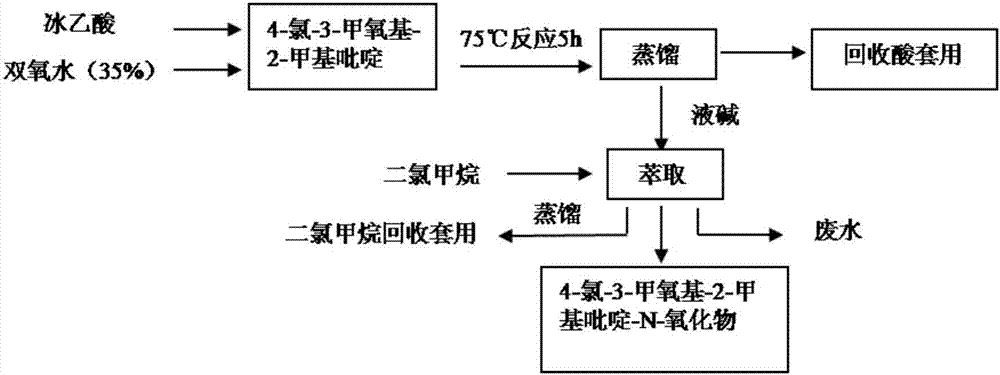
Synthesis method of 4-chloro-3-methoxy-2-methylpyridine-N-oxide
A technology of methyl pyridine and synthesis method, applied in directions such as organic chemistry, can solve problems such as poor catalytic effect, low yield of pyridine-N-oxide, etc., achieve mild reaction, improve oxidation reaction effect, and reduce the effect of raw material usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
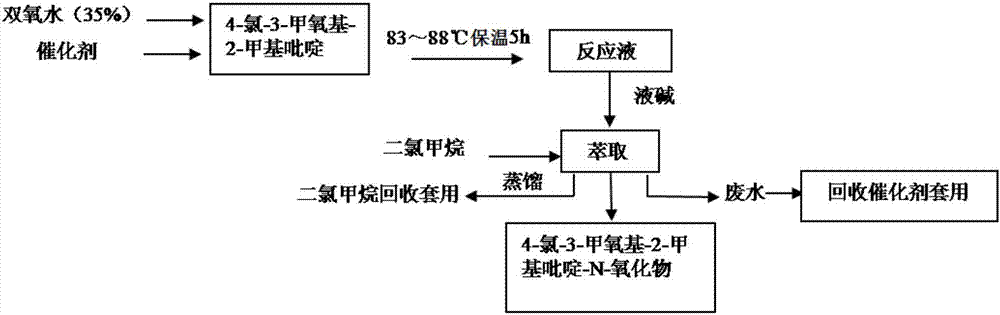
[0027] A kind of synthetic method of Pandoprazole intermediate 4-chloro-3-methoxyl group-2-picoline-N-oxide compound of the present invention, such as image 3 As shown, follow the steps below:
[0028] Step 1, dissolving 10 parts by weight of phosphotungstic acid in neutral water to obtain a 20% phosphotungstic acid solution;
[0029] Step 2, weigh 250 parts by weight of 4-chloro-3-methoxy-2-picoline, slowly add the phosphotungstic acid solution prepared in step 1 under stirring, stir until completely mixed, and heat in a water bath to raise the temperature To 90°C, 300 parts by weight of hydrogen peroxide with a mass concentration of 35% was added dropwise, and the dropping rate of hydrogen peroxide was 50 parts by weight / hour; and then kept at 83°C for 5 hours to obtain a reaction solution;
[0030] Step 3, after cooling the reaction solution to 25°C, add a dilute alkali solution (aqueous sodium hydroxide solution with a mass concentration of 15%) to adjust the pH to 7-9, ...
Embodiment 2
[0032] A kind of synthetic method of Pandoprazole intermediate 4-chloro-3-methoxyl group-2-picoline-N-oxide of the present invention, specifically carry out according to the following steps:
[0033] Step 1, dissolving 12 parts by weight of phosphotungstic acid in weakly acidic water to obtain a 25% mass concentration of phosphotungstic acid solution;
[0034]Step 2, weigh 220 parts by weight of 4-chloro-3-methoxy-2-methylpyridine, slowly add the phosphotungstic acid solution prepared in step 1 under stirring, stir until completely mixed, and heat up in a water bath To 87°C, 350 parts by weight of hydrogen peroxide with a mass concentration of 35% was added dropwise, and the dropping rate of hydrogen peroxide was 60 parts by weight / hour; and then kept at 85°C for 5 hours to obtain a reaction solution;
[0035] Step 3, after cooling the reaction solution to 30°C, add a dilute alkali solution (aqueous sodium hydroxide solution with a mass concentration of 12%) to adjust the pH t...
Embodiment 3
[0037] A kind of synthetic method of Pandoprazole intermediate 4-chloro-3-methoxyl group-2-picoline-N-oxide of the present invention, specifically carry out according to the following steps:
[0038] Step 1, dissolving 13 parts by weight of phosphotungstic acid with weakly acidic water to obtain a 30% mass concentration of phosphotungstic acid solution;
[0039] Step 2, weigh 200 parts by weight of 4-chloro-3-methoxy-2-picoline, slowly add the phosphotungstic acid solution prepared in step 1 under stirring, stir until completely mixed, and heat the water bath to raise the temperature To 85°C, 380 parts by weight of hydrogen peroxide with a mass concentration of 35% was added dropwise, and the dropping rate of hydrogen peroxide was 65 parts by weight / hour; and then kept at 88°C for 5 hours to obtain a reaction solution;
[0040] Step 3, after cooling the reaction solution to 40°C, add a dilute alkaline solution (8% sodium hydroxide aqueous solution) to adjust the pH to 7-9, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com