A kind of n-aryl-2-thiophene amide derivatives and its preparation method and application
A technology of use and thiophene carboxylic acid, applied in the field of medicine, can solve the problems of inability to meet the needs of patients, the function of pancreatic islet beta cells is attenuated, and the blood sugar of patients is not up to the standard.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of embodiment 1N-(2-(4-chlorobenzoyl) phenyl)-5-bromothiophene-2-carboxamide Y1
[0029] Add 5-bromo-2-thiophenecarboxylic acid (1mmol, 206mg), 3mL thionyl chloride, and 3 drops of pyridine into a 50mL round-bottomed flask, stir and add flow (80°C), after 2 hours, stop the reaction and rotate The solvent was removed by evaporation to obtain 5-bromo-2-thienyl chloride, which was added to 5 mL of dry dichloromethane for later use. Add 2-amino-4'-chlorobenzophenone (1 mmol, 232 mg), 5 mL of dichloromethane, and 1 mL of triethylamine into a 50 mL round bottom flask, stir to dissolve, and cool to 0° C. in an ice-water bath. Under stirring, a dichloromethane solution of 5-bromo-2-thiophene acid chloride was added dropwise (dropped within 10 minutes), and after the dropwise addition was completed, the stirring reaction was continued for 2 hours. Stop the reaction, remove the solvent under reduced pressure with a rotary evaporator, and separate and purify ...
Embodiment 2
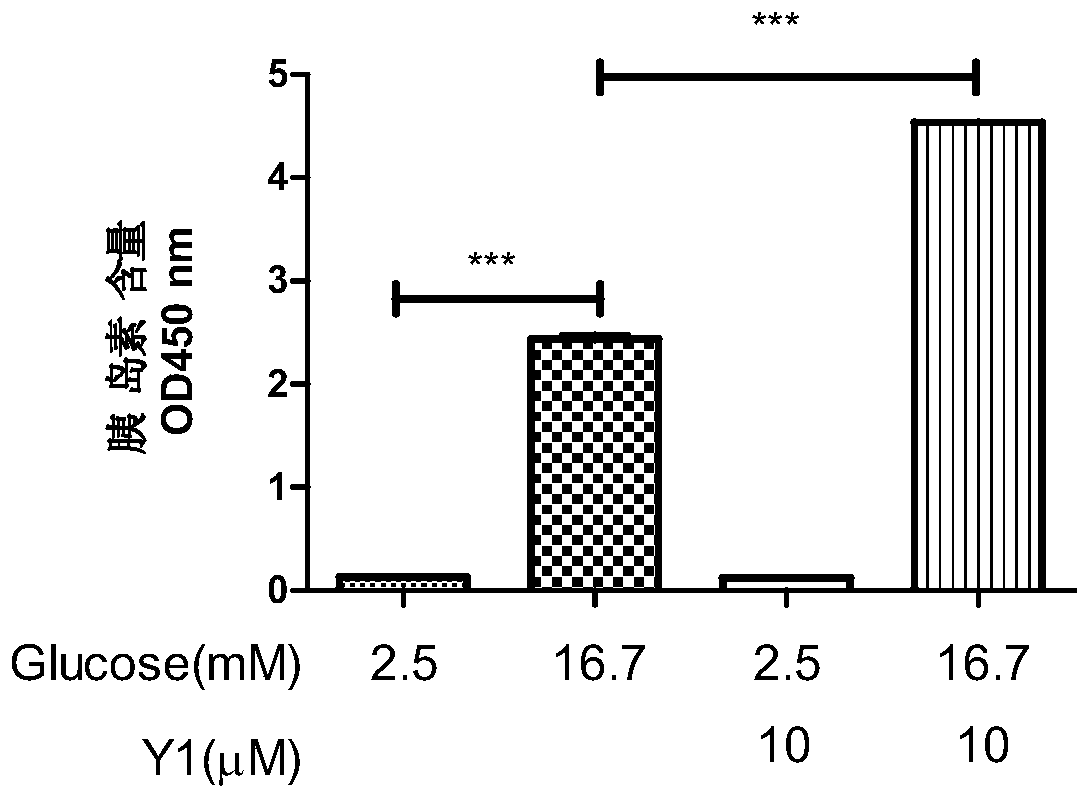
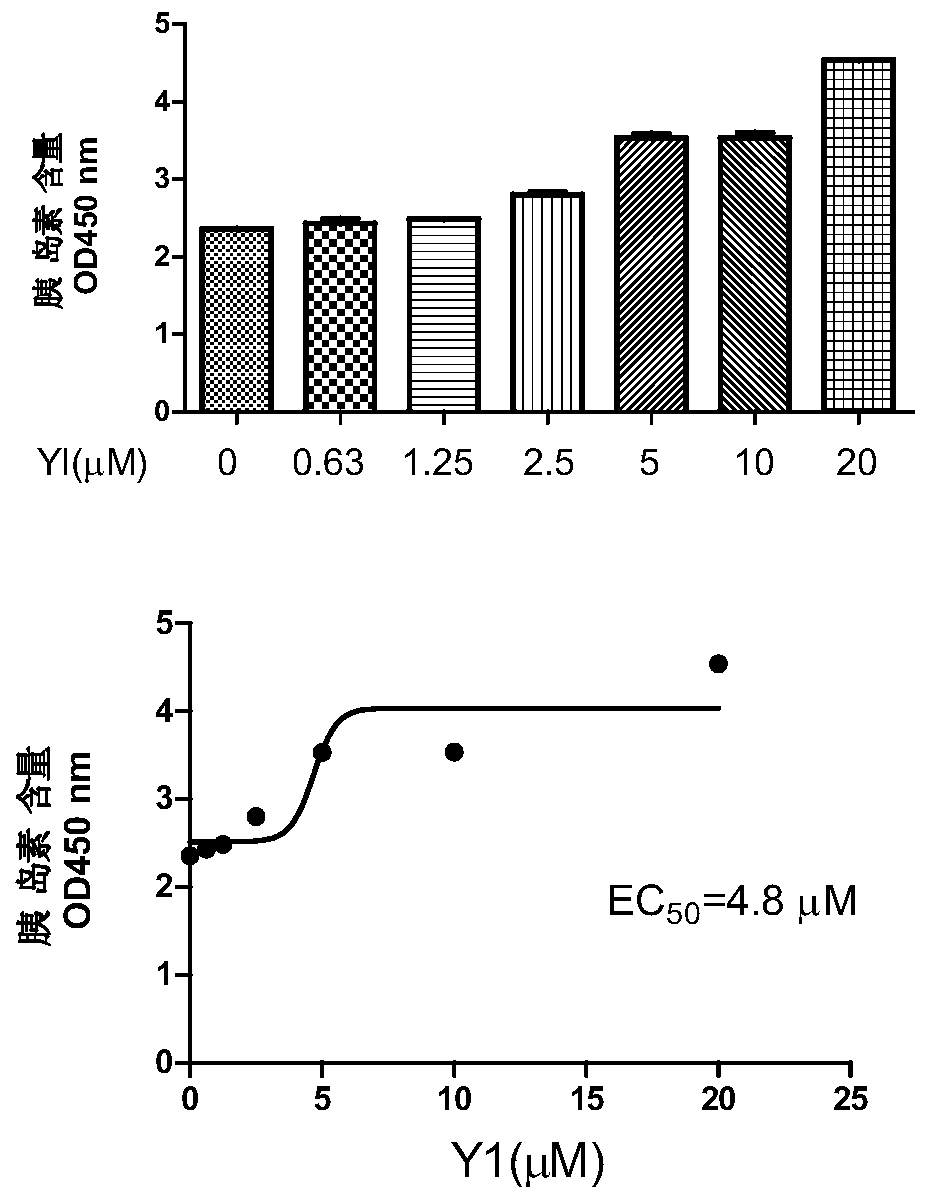
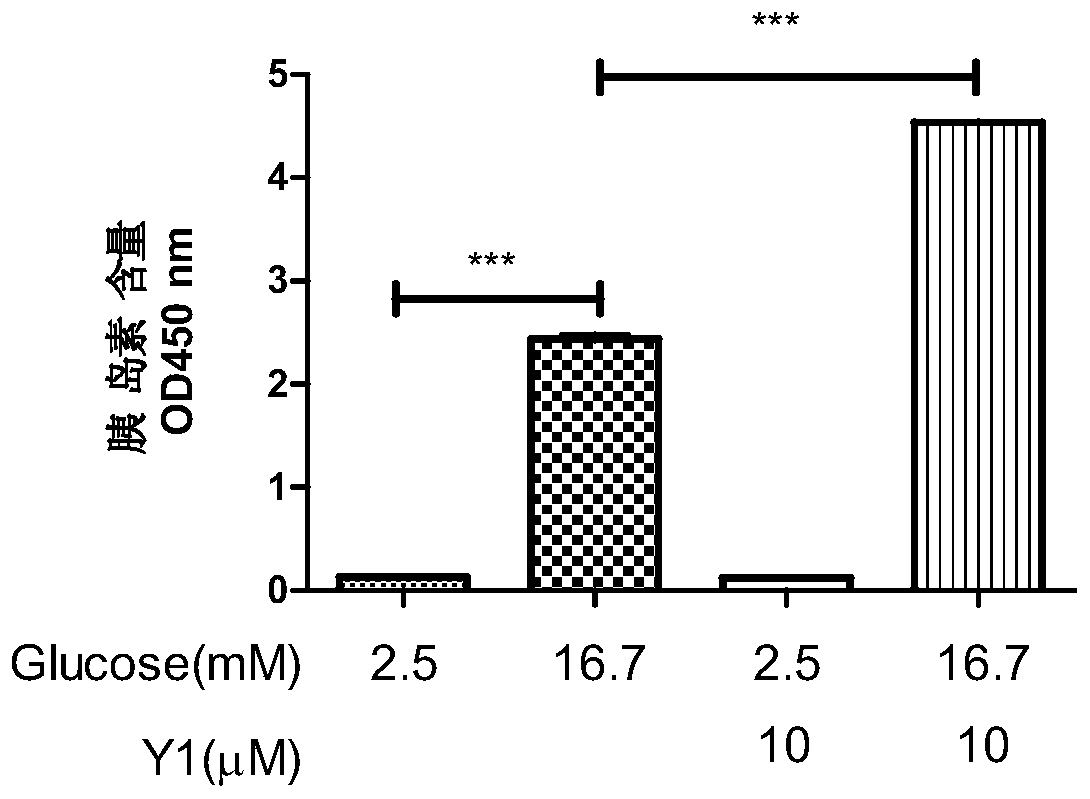
[0031] Example 2 Detection of insulin secretion-stimulating activity of N-(2-(4-chlorobenzoyl)phenyl)-5-bromothiophene-2-carboxamide Y1
[0032] Insulin-stimulating activity was detected by rat insulinoma cells INS-832 / 13 cells. INS-832 / 13 cells were cultured in RPMI1640 medium containing 10% fetal bovine serum, 10mM hydroxyethylpiperazine ethanesulfonic acid, 2mM L-glutamate, 1mM sodium pyruvate, 0.05mM mercaptoethanol, placed Contains 5% CO 2 cultured in a cell culture incubator.
[0033] INS-832 / 13 cells take 10 5 Plate / well in 24-well plate, 37°C, 5% CO 2 Incubate for 48 hours. When the cells grow well, suck out the RPMI1640 medium, add sugar-free buffer solution (115mM sodium chloride, 5mM potassium chloride, 24mM sodium bicarbonate, 2.5mM calcium chloride, 1mM magnesium chloride, 10mM hydroxyethylpiperazine ethylsulfur acid, 0.1% bovine serum albumin, pH7.2) for 2 hours, and then replaced with buffer solutions containing 2.5mM and 16.7mM glucose concentrations and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com