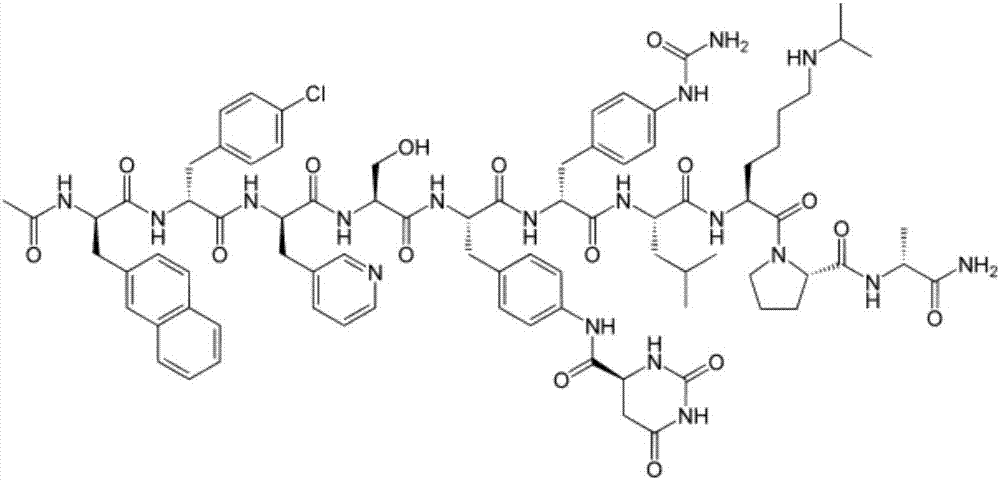
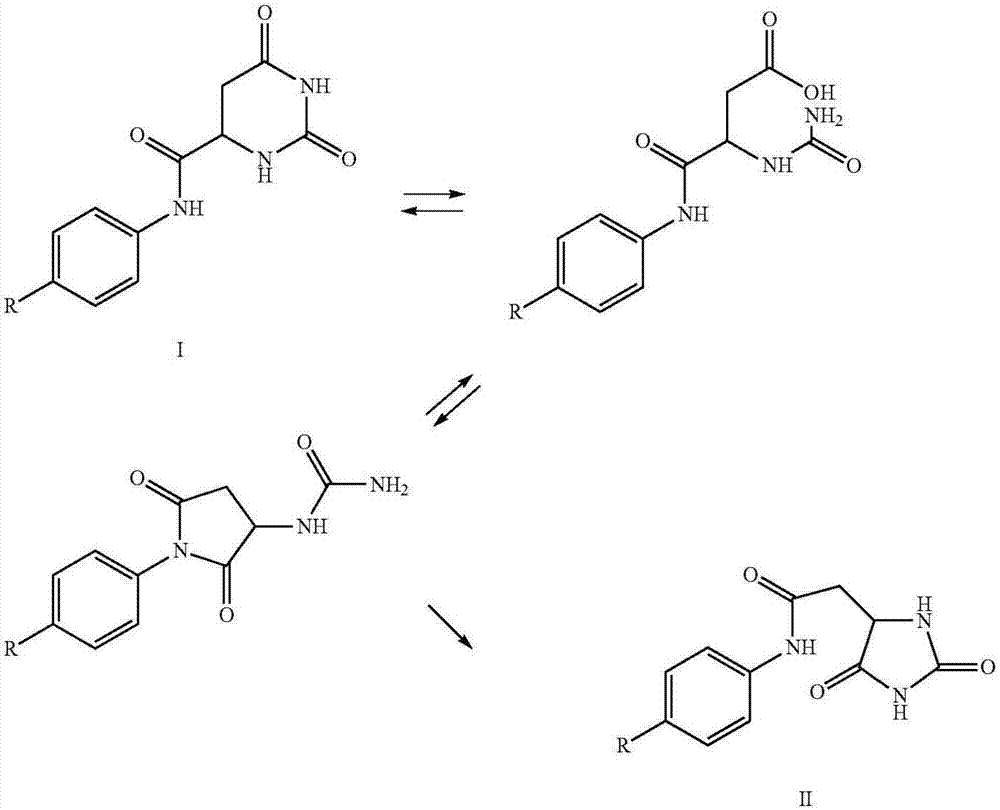
Method for preparing degarelix by using solid and liquid combination
A degarelix and synthetic method technology, applied in the field of peptide synthesis, can solve the problems of high operator requirements, complicated operation, troublesome post-processing, etc., and achieve the effects of cheap and easy-to-obtain raw materials, easy to scale up production, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Synthesis of Ac-D-2Nal-D-4Cpa-D-3Pal-Ser(tBu)-4Aph(Fmoc)-OH
[0066] Accurately weigh 199.6g (1.0mol) of H-D-4Cpa-OH and 127.2g (1.2mol) of sodium carbonate and dissolve them in 2400mL of water, slowly add Ac-D-2Nal-OSu THF solution (298.3 g, 1.0mol) / 2000ml, stirred and reacted, TLC monitored the reaction end point, after the reaction was complete, THF was evaporated off by rotary evaporation, and dilute hydrochloric acid was added in an ice-water bath to adjust the pH value of the solution to 2~3, extracted 3 times with 2000ml ethyl acetate, combined with organic phase, washed 3 times with 400ml of saturated brine, dried over anhydrous sodium sulfate, concentrated to 2000ml by rotary evaporation, and stood for crystallization to obtain 390.3g of Ac-D-2Nal-D-4Cpa-OH, with a yield of 82.1%.
[0067] Accurately weigh 136.3g (0.82mol) of H-D-3Pal-OH and 106.0g (1.0mol) of sodium carbonate and dissolve in 2400mL of water, slowly add Ac-D-2Nal-D-4Cpa-OSu at low te...
Embodiment 2
[0070] Example 2: Synthesis of Ac-D-2Nal-D-4Cpa-D-3Pal-Ser(tBu)-4Aph(Fmoc)-OH
[0071] Weigh 500.0g (sub=1.0mmol / g) of CTC resin and place it in a synthesis column, wash twice with 4000mL DMF, add 4000mL DCM to swell for 30min; (tBu)-OH DCM / DMF (3 / 1, volume ratio) solution 1500ml, after stirring, add DIPEA 330ml (2000mmol), drum N 2 React for 60min, remove the reaction solution, add DCM / CH 3OH / DIPEA (volume ratio 17:2:1) mixed solution 3000ml capped 3 times, 10min each time; then washed 6 times with DMF, took a small sample to measure the degree of substitution was 0.75mmol / g, the synthesis scale was 0.5mol.
[0072] The resin in the synthesis column was deprotected twice by 3000ml 20% piperidine / DMF solution, washed 6 times with DMF; then added the activated Fmoc-D-3Pal-OH / HOBT / DIC solution, reacted at room temperature for 3h, filtered off After the reaction solution, wash 6 times with DMF; then recycle the above operation, respectively react with Fmoc-D-4Cpa-OH and Ac-D-2N...
Embodiment 3
[0075] Example 3: Preparation of H-D-4Aph(Cbm)-Leu-Lys(Boc, iPr)-Pro-D-Ala-NH-Resins
[0076] Accurately weigh 220 g of RinkAmideAM resin (initial substitution degree 0.91 mmol / g) and place it in a peptide resin synthesis reactor, add 1.0 LDCM to swell for 2 h. After the swelling is completed, wash with DMF three times, 1.0 L each time, and then add 2.0 L of 20% piperidine / DMF solution for deprotection twice, for 10 min and 10 min respectively. After the deprotection was completed, the resin was washed 6 times with DMF, 2.0 L each time. Weigh 124.4g (0.40mol) of Fmoc-D-Ala-OH, 59.4g (0.44mol) of HOBt and dissolve them in 0.8L DMF, add 68mL (0.44mol) of DIC to activate, add the solution into the reactor, react for 2h, The Kaiser test monitors the progress of the reaction. After the reaction, the resin was washed 6 times with DMF. Then repeat the above operation, and sequentially react with Fmoc-Pro-OH, Fmoc-Lys(Boc, iPr)-OH, Fmoc-Leu-OH to prepare H-Leu-Lys(Boc, iPr)-Pro-D- ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com