Carboline and carbazole based imaging agents for detecting neurological dysfunction
An alkyl, aryl-based technology in the field of carboline- and carbazole-based imaging agents for the detection of neurological dysfunction, which can solve problems such as accurate distinctions not expected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
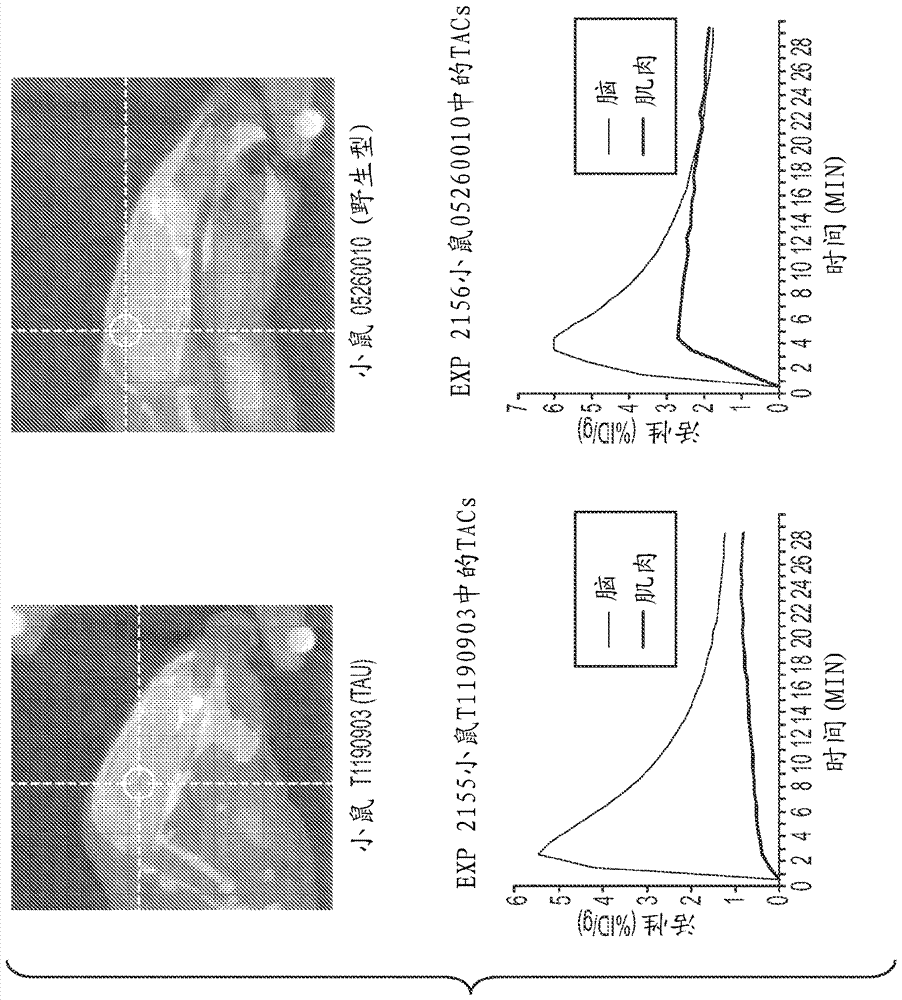
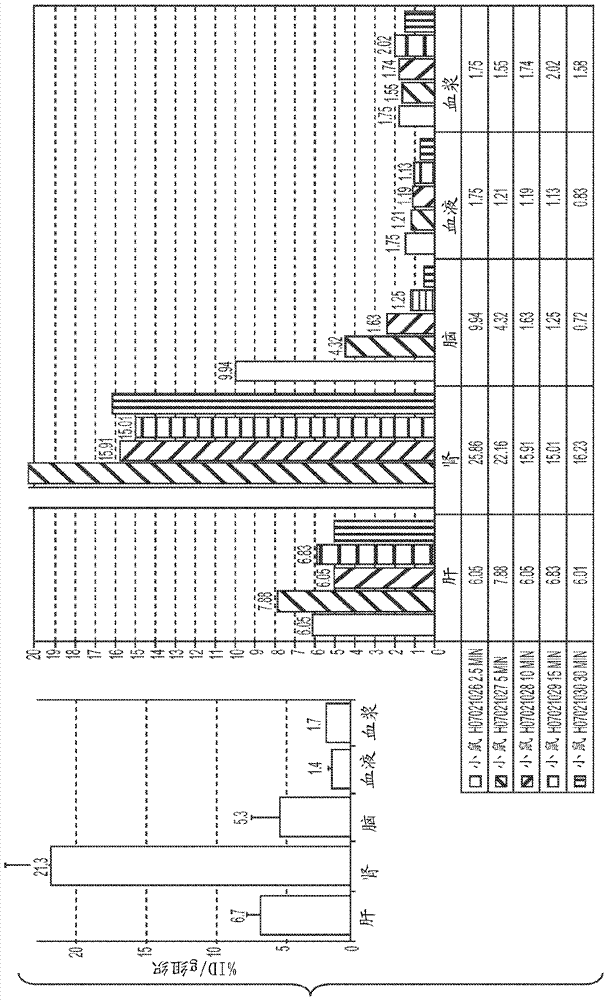
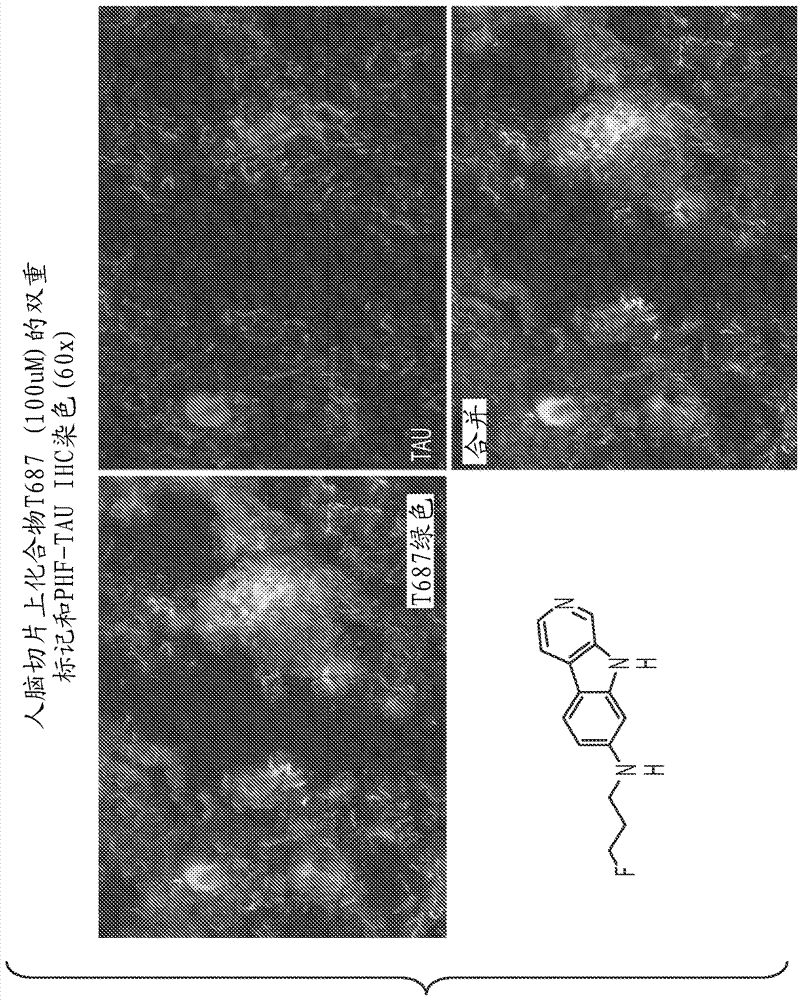
[0094] detail
[0095] "Halogen" or "halo" means F, Cl, Br and I.
[0096] "Alkyl" means a saturated monovalent hydrocarbon radical having straight or branched chain portions. Examples of alkyl groups include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, and tert-butyl.
[0097] "Alkenyl" means an alkyl moiety having at least one carbon-carbon double bond, wherein alkyl is as defined above. Examples of alkenyl groups include, but are not limited to, ethenyl and propenyl.
[0098] "Alkynyl" means an alkyl moiety having at least one carbon-carbon triple bond, wherein alkyl is as defined above. Examples of alkynyl include, but are not limited to, ethynyl and 2-propynyl.
[0099] "Alkylene" or "alkenylene" means a saturated divalent hydrocarbon radical having a straight or branched chain portion, ie, typically present as a bridging or linking group between two other groups. Examples of alkylene include -CH 2 -(methylene); -CH 2 CH 2 -(ethylene);-CH 2 CH 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com