Water indissolvable/micro-dissolvable drug sustained release composition
A slow-release composition, poorly water-soluble technology, used in drug delivery, pharmaceutical formulations, nanocapsules, etc., can solve the problems of inconvenient clinical use and poor patient compliance, maintain effective blood drug concentrations, reduce drug delivery Frequency, the effect of convenient life and work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
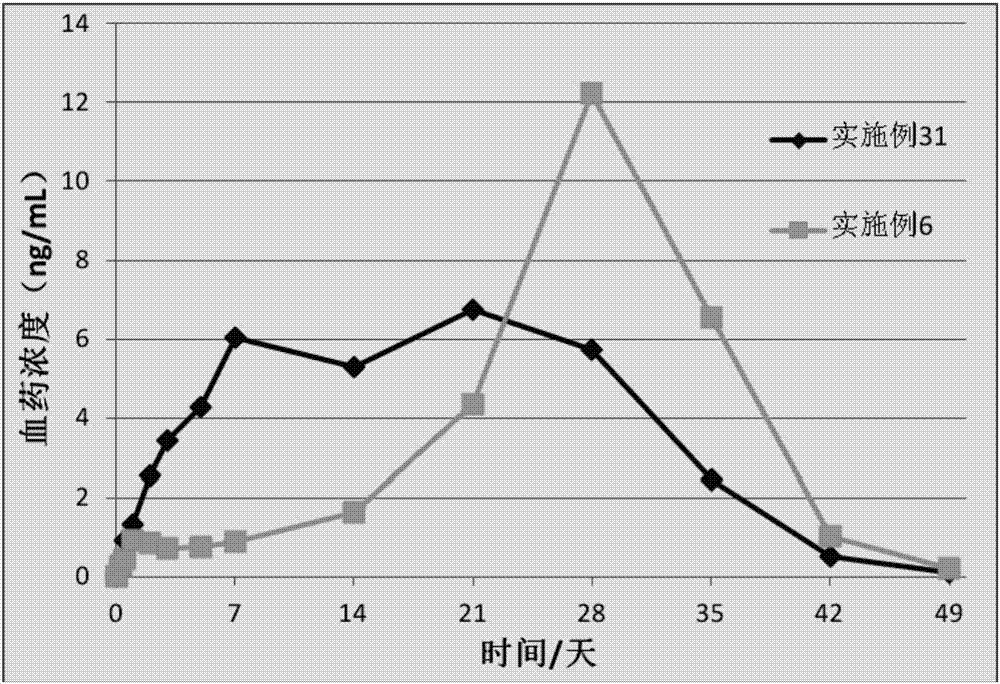
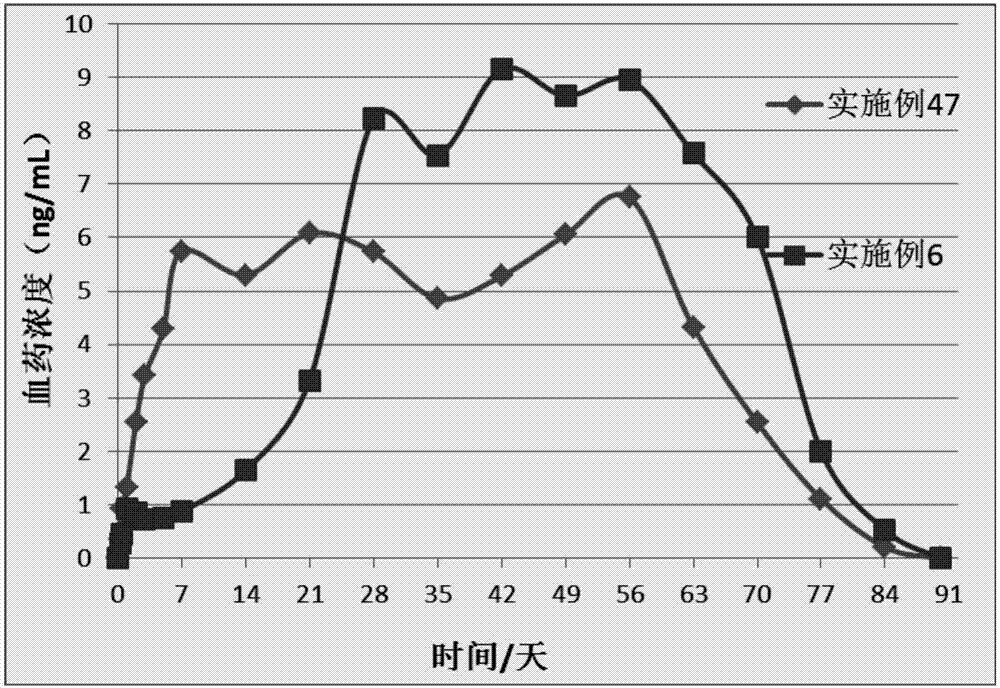
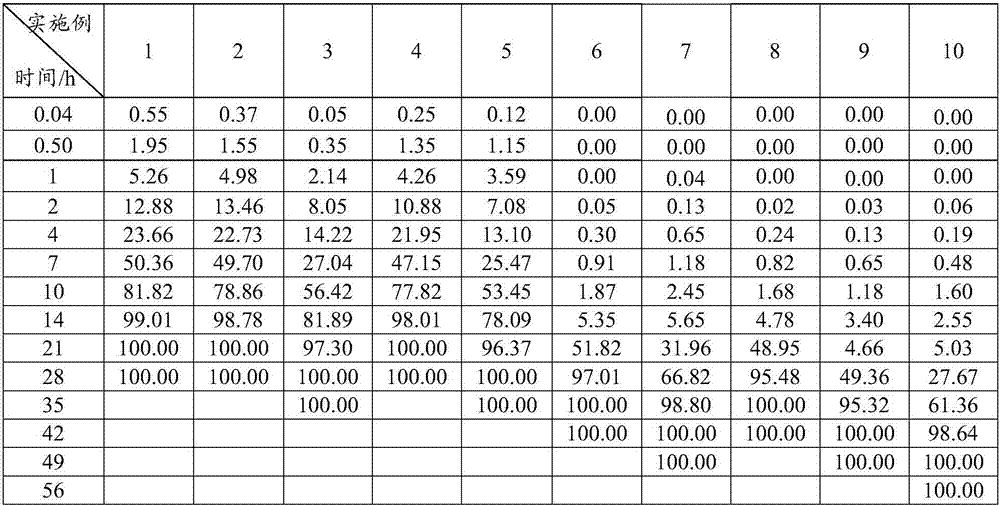
[0063] A kind of sustained-release microsphere (sustained-release microsphere A) of this embodiment has no obvious delayed release period or a short delayed release period, and the drug release time is 2-3 weeks, and its preparation raw materials include the following parts by weight Components: 30 parts of poorly water-soluble / slightly soluble drugs, 70 parts of poorly water-soluble polymers; the poorly water-soluble / slightly soluble drugs are risperidone, the poorly water-soluble polymers are PLGA, and the The weight average molecular weight is 20kDa, the viscosity is 0.23dL / g, the molar ratio of lactide to glycolide is 70:30, and it has a carboxyl terminal.
[0064] The preparation method of the slow-release microspheres described in this example is: dissolving the poorly water-soluble / slightly soluble drug and the poorly water-soluble polymer in methylene chloride 10 times the mass to obtain a clear oil inner phase; then the oil The internal phase solution was added to the...
Embodiment 2
[0067] A kind of sustained-release microsphere (sustained-release microsphere A) of the present embodiment has no obvious release delay period and the drug release time is 2 weeks, and its preparation raw materials include the following components by weight: insoluble in water / 35 parts of slightly soluble drugs, 65 parts of poorly water-soluble polymers; the poorly water-soluble / slightly soluble drugs are risperidone, the poorly water-soluble polymers are PLGA, and the weight-average molecular weight of the PLGA is 25kDa, and the viscosity is 0.28dL / g, wherein the molar ratio of lactide to glycolide is 75:25, which has a terminal carboxyl group.
[0068] The preparation method of the slow-release microspheres described in this example is the same as that in Example 1.
[0069] The obtained sustained-release microspheres are round in shape, smooth in surface, and the particle diameter is 24-100 μm. The drug loading rate is 31.94% and the drug encapsulation rate is 91.26%.
Embodiment 3
[0071] A kind of sustained-release microsphere (sustained-release microsphere A) of the present embodiment has no obvious release delay period, and the drug release time is 3 weeks. The raw materials for the preparation of the sustained-release microsphere include the following components by weight: Divide: 35 parts of poorly water-soluble / slightly soluble drugs, 55 parts of poorly water-soluble polymers; the poorly water-soluble / slightly soluble drugs are risperidone, the poorly water-soluble polymers are PLGA, and the weight average of The molecular weight is 30kDa, the viscosity is 0.32dL / g, the molar ratio of lactide to glycolide is 80:20, and it has a terminal carboxyl group.
[0072] The preparation method of the slow-release microspheres A described in this example is the same as that in Example 1.
[0073] The obtained slow-release microsphere A has a round shape, a smooth surface, a particle diameter of 20-95 μm, a drug loading rate of 40.89%, and a drug encapsulation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com