Composition and purpose of deoxycholic acid, and methods for the purification of deoxycholic acid
A pharmaceutical composition and application technology, applied in the field of synthesis of deoxycholic acid or its salt, intermediates in the synthesis of deoxycholic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
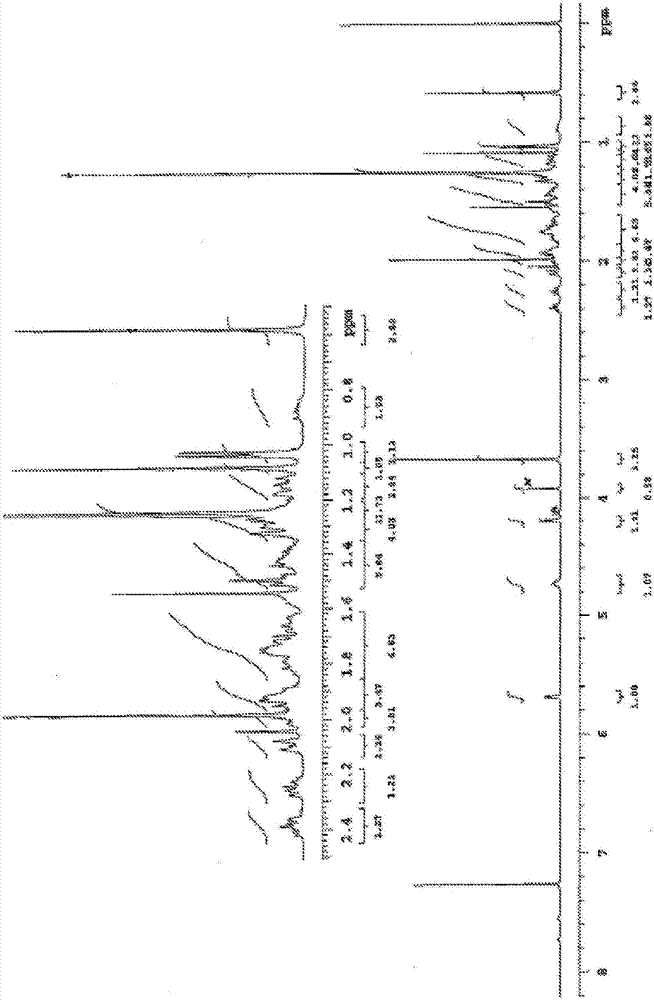
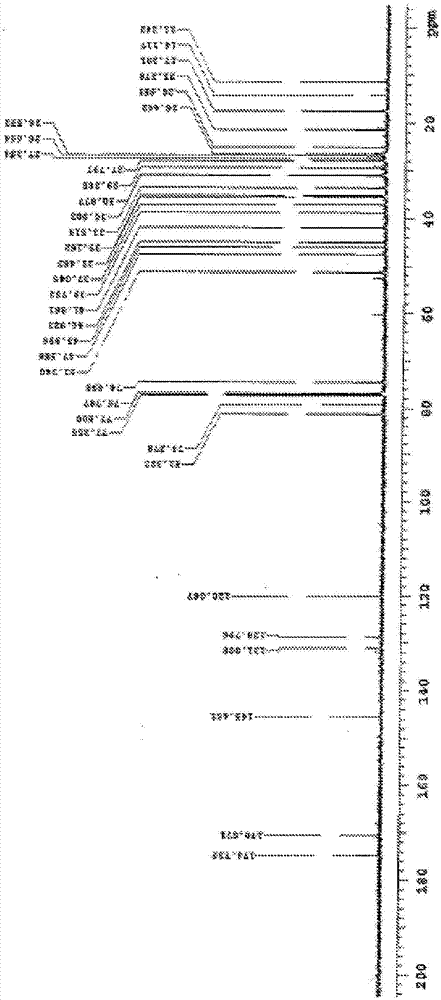
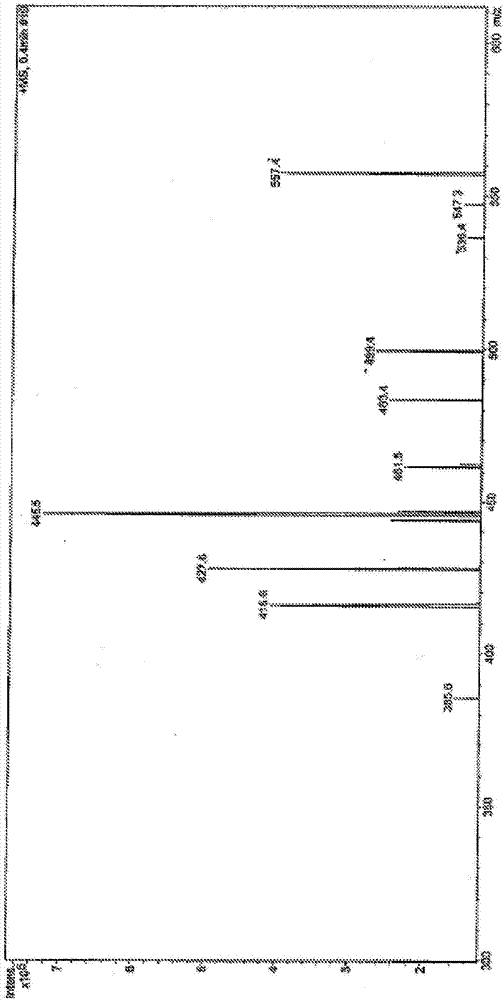
Image
Examples
example 1
[0557] Synthesis of 3α-Acetoxy-5β-androstane-9,11-en-17-one from Hydrocortisone (36)
[0558] step 1
[0559]
[0560] To a solution of hydrocortisone (25.0 g) in DMF (150 mL) was added 10% Pd / C (1.5 g, 6-wt%) and the resulting slurry was autoclaved (60 psi) at 25-35 °C Hydrogenated for 6 hours. After complete disappearance of the starting material was demonstrated by TLC (30% EtOAc in DCM), The crude reaction mixture was filtered over a (8 g) bed and washed with DMF (100 mL). The solvent was completely removed by vacuum distillation below 65 °C to afford compound 15 (23.0 g, 91.5%) as a white solid.
[0561] step 2
[0562]
[0563] To a solution of compound 15 (23.0 g) in ethanol (350 mL) and DCM (350 mL) was added sodium borohydride (2.4 g), and the resulting solution was stirred at 25-35 °C for 3 hours. At this point, 50% acetone in water (200 mL) was added to quench excess reagent, followed by sodium periodate (33.7 g). The resulting solution was stirred at 2...
example 2
[0571] (Z)-3α-Acetoxy-5β-pregna-9(11),17(20)-diene(30):
[0572]
[0573] Compound 30 can be prepared by converting compound 28 to compound 30 using a procedure similar to that described in Example 8.
[0574] Methods and examples of the preparation and purification of DCA from compound 60 are described in GB2452358 and in U.S. provisional application 61 / 288,132 entitled "METHODS FOR THE PURIFICATION OF DEOXYCHOLICACID" filed on December 18, 2009 , both of which are incorporated herein by reference in their entirety.
example 3
[0576] Impurities isolated during the preparation of DCA or its intermediates
[0577] The following compounds were recovered as impurities during the synthesis described herein:
[0578]
[0579] The above compound has utility as an intermediate that can be recycled into the reaction scheme leading to compound 18. For example, compounds 61 and 62 can be dehydrogenated by conventional means to give 4,5-ene compounds, which can then be hydrogenated to achieve the appropriate stereochemistry at the 5-position.
[0580] The 17-keto group of compounds 63 and 65 can be protected by conventional means such as ketal formation. The 3-hydroxyl groups of compounds 63 and 65 can then be oxidized to form 3-keto groups. For compound 63, dehydrogenation at the 4,5 positions followed by hydrogenation will achieve the appropriate stereochemistry at the 5-position. For compounds 63 and 65, reduction of the 3-keto group followed by deprotection of the 17-keto group affords compound 18.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com