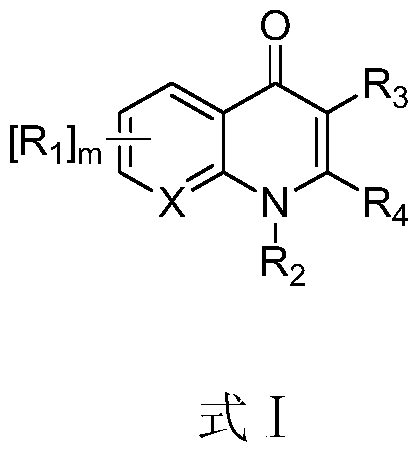
A compound and its application in the preparation of anti-hepatitis C virus medicine
A compound and drug technology, applied in the application field of preparing anti-HCV drugs, can solve the problems of easy mutation of the virus, failure to benefit patients, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
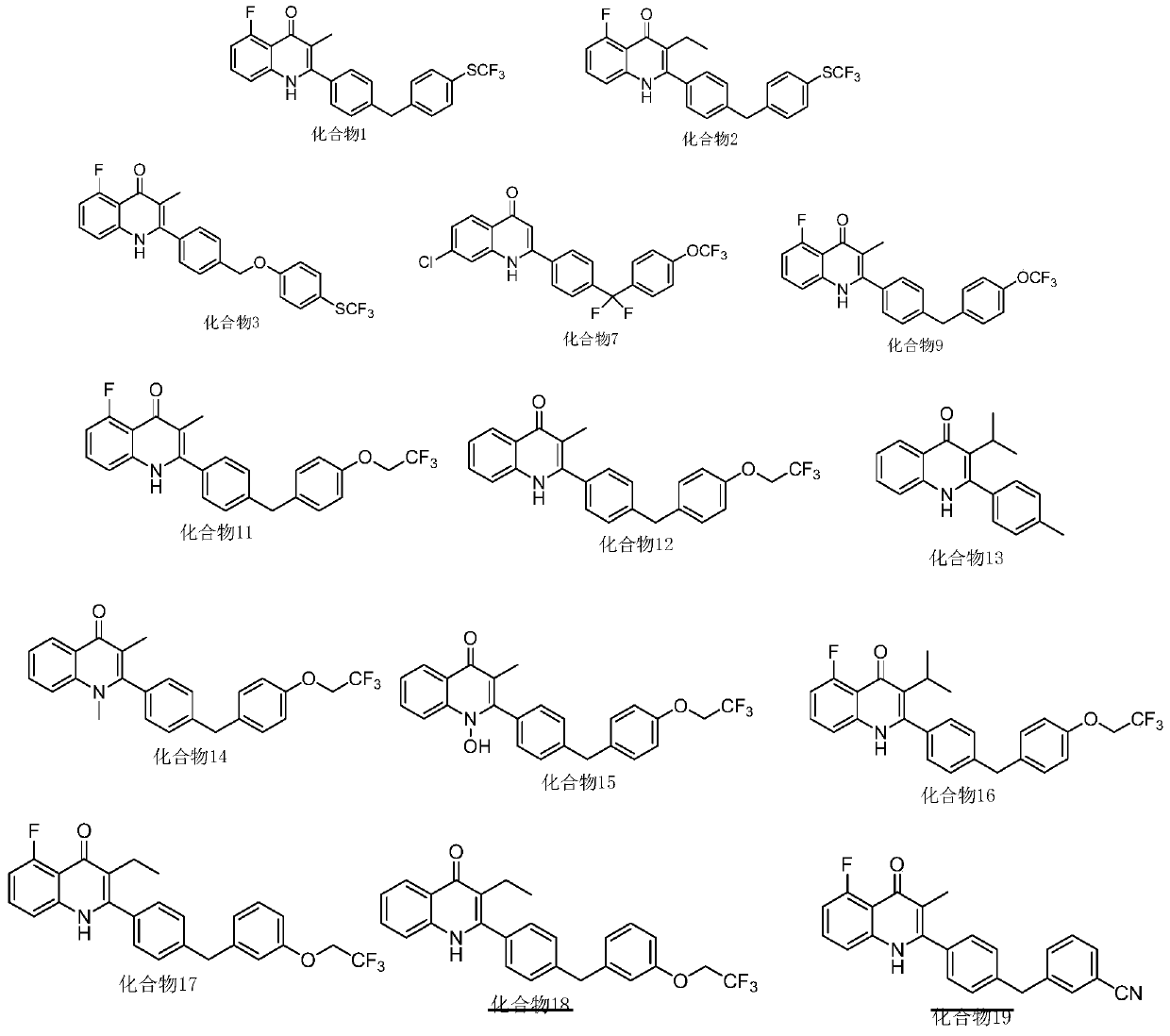
[0123] Embodiment 1, the preparation of compound 1
[0124]
[0125] Get intermediate A1 in 100ml round bottom flask, add 198mg Pd(dppf) 2 .DCM, 2g potassium phosphate and 1.2g p-trifluoromethylthiophenylboronic acid pinacol ester, add 10ml toluene, under the protection of argon, react at 100°C for 12h, spin the solvent, add 100ml water, extract with 20ml dichloromethane Twice, using petroleum ether: ethyl acetate = 30:1 to pass through a silica gel column to obtain 1 g of intermediate A3 (colorless liquid), with a yield of 64%.
[0126] Take 1g of intermediate A3 and 650mg of intermediate A4 in a 25ml round bottom flask, add 240mg of p-toluenesulfonic acid monohydrate and 10ml of n-butanol, react at 130°C for 16h, spin the solvent under reduced pressure, add water, and use 20ml of ethyl acetate Extracted twice, and passed through a silica gel column with ethyl acetate:petroleum ether=1:1 to obtain 320 mg of compound 1 (white solid), with a total yield of 24%.
[0127] Th...
Embodiment 2
[0129] Embodiment 2, the preparation of compound 2
[0130]
[0131] Referring to Example 1 where A1 was replaced by A5, other conditions and subsequent steps were the same to obtain compound 2.
[0132] The characterization data of compound 2 are as follows:
[0133] H-NMR (400MHz, d 6 -DMSO, ppm): 0.93(t, J=6.96Hz, 3H), 2.26(q, J=7.12Hz, 2H), 4.14(s, 1H), 6.91(dd, J=8.12Hz, J=11.28Hz ,1H),7.34(d,J=8.32Hz,1H),7.45–7.54(m,7H),7.67(d,J=7.68Hz,2H),11.52(s,1H).LC-MS: calcd for C 25 h 20 f 4 NOS[M+H] + :458.11, found 458.21.
Embodiment 3
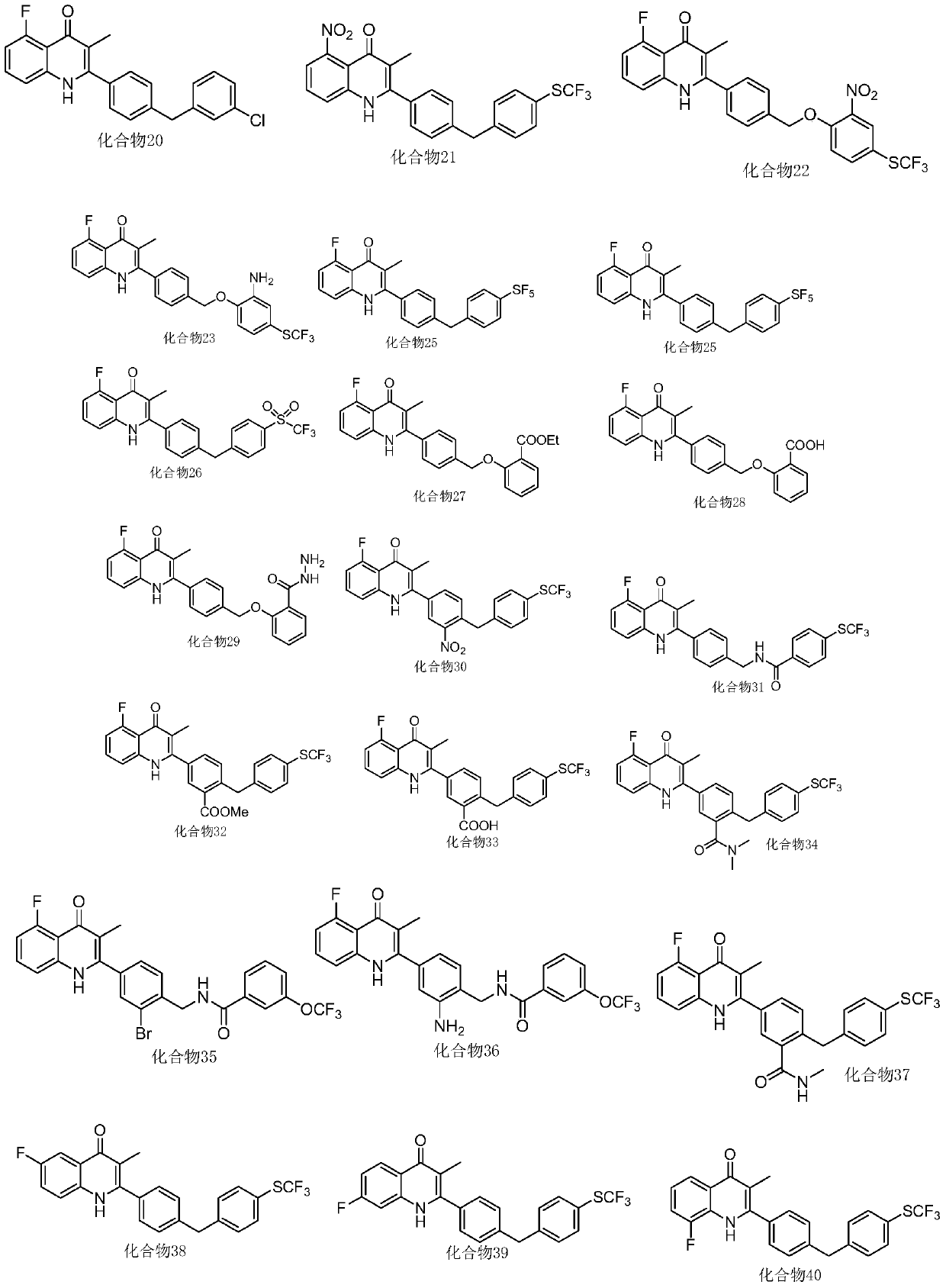
[0134] Embodiment 3, the preparation of compound 3
[0135]
[0136] Take 600mg of intermediate A1 in a 100ml round bottom flask, add 420mg of potassium carbonate and 600mg of p-trifluoromethylthiophenol, add 10ml of N,N-methylformamide DMF, and react at 60°C for 3h under the protection of argon, add 100ml of water was extracted twice with 20ml of dichloromethane and passed through a silica gel column with petroleum ether: ethyl acetate = 20:1 to obtain 918mg of intermediate A6 (colorless liquid) with a yield of 90%.
[0137] Referring to the steps and conditions in Example 1, A3 was replaced by A6, and other conditions were the same to obtain compound 3.
[0138] The characterization data of compound 3 are as follows:
[0139] 1 H-NMR (400MHz, d 6 -DMSO,ppm):11.62(s,1H),7.66(m,4H),7.58(d,J=7.96Hz,2H),7.52(m,1H),7.39(d,J=8.40Hz,1H) ,7.20(d,J=8.56Hz,2H),6.94(dd,J=11.88Hz,J=7.96Hz,1H),5.30(s,2H),1.4(s,3H).LC-MS: calcd for C 24 h 18 f 4 NO 2 S[M+H] + :460.09, found 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com