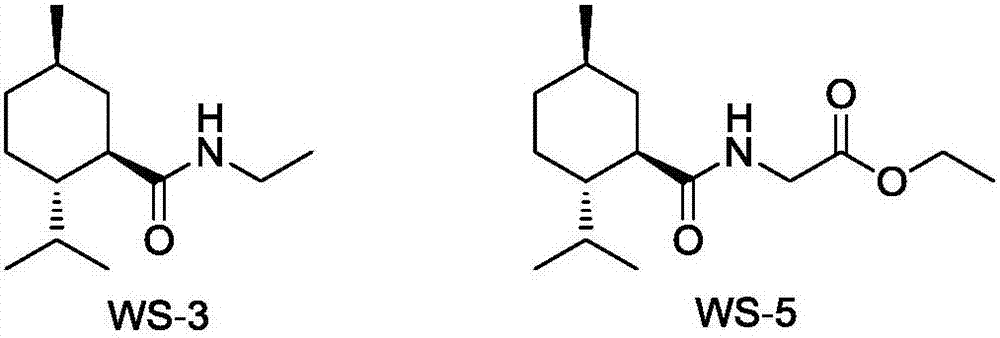
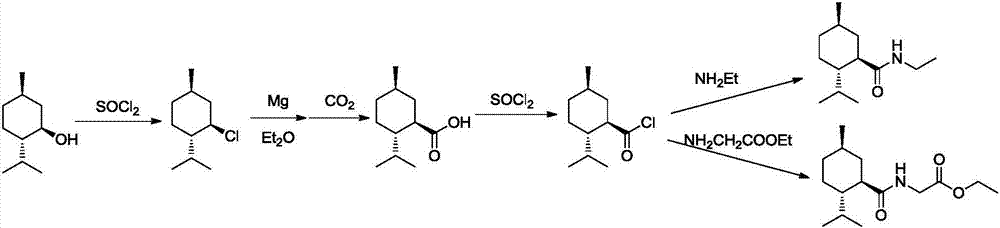
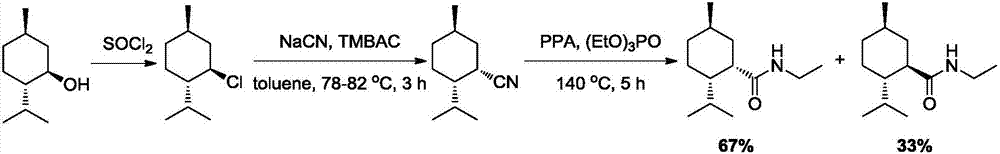
Preparation method of L-N-ethyl-p-menthane-3-carboxamide compound
A technology of menthol amide and amine compound, applied in the field of preparation of L-menthol amide compound, can solve the problems of strong pungent smell, burning sensation, expensive menthol and the like, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Friedel-Crafts reaction
[0052] At 0-5°C, AlCl 3 (365g, 2.74mol) was added in 1000mL of nitromethane, passed into phosgene (542g, 5.48mol) and then insulated and stirred for 0.5 hour, then cymene (335g, 2.5mol) was added dropwise, and the dropwise process controlled the reaction temperature at 0 -5°C, after the dropwise addition, keep it warm for about 5 hours until no HCl gas is generated. After the reaction, remove excess phosgene and solvent, distill under reduced pressure, collect 67-70°C / 200pa fraction to obtain 2-isopropyl-5-methylbenzoyl chloride, 300g, yield 61%.
[0053] The analysis result of 2-isopropyl-5-methylbenzoyl chloride: 1H NMR (400MHz, CDCl 3 )δ7.93(s,1H),7.54(s,1H),7.43(s,1H),2.87(s,1H),2.42(s,3H),1.17(s,6H), HRMS(ESI)m / z[M+Na]+: calculated for[C 11 h 13 ClNaO]+: 219.0553. Found: 219.0540.
[0054] (2) Condensation reaction
[0055] At 0°C, 70wt% ethylamine aqueous solution (102g, 1.6mol) and 20wt% NaOH (320g, 1.6mol) aqueous solution w...
Embodiment 2
[0063] (1) Friedel-Crafts reaction
[0064] At 0-5°C, AlCl 3 (166g, 1.25mol) was added in 350mL dimethyl sulfoxide, passed into phosgene (542g, 5.48mol) and kept stirring for 0.5 hours, then added dropwise cymene (335g, 2.5mol), and the dropping process controlled the reaction temperature At 0-5°C, after the dropwise addition, keep the reaction for about 10 hours until no HCl gas is generated. After the reaction, remove excess phosgene and solvent, distill under reduced pressure, collect 67-70°C / 200pa fraction to obtain 2-isopropyl-5-methylbenzoyl chloride, 290g, yield 59%.
[0065] (2) Condensation reaction
[0066] At 0°C, 70wt% ethylamine aqueous solution (99g, 1.54mol) and 5wt% NaOH (1220g, 1.53mol) aqueous solution were mixed, then 2-isopropyl-5-methylbenzoyl chloride (300g, 1.5mol) was added dropwise ) in anisole solution (350 mL). After the reaction, wash with water (300mL*3), distill under reduced pressure, collect the 95-98℃ / 150pa fraction to obtain N-ethyl-2-isop...
Embodiment 3
[0072] (1) Friedel-Crafts reaction
[0073] At 0-5°C, AlCl 3 (666g, 5mol) was added in 3000mL nitrobenzene, passed into phosgene (742g, 7.5mol) and then insulated and stirred for 0.5 hour, then cymene (335g, 2.5mol) was added dropwise, and the reaction temperature was controlled at 0- 5°C, keep the reaction for about 2 hours after the dropwise addition until no HCl gas is generated. After the reaction, remove excess phosgene and solvent, distill under reduced pressure, collect 67-70°C / 200pa fraction to obtain 2-isopropyl-5-methylbenzoyl chloride, 304g, yield 62%.
[0074] (2) Condensation reaction
[0075]At 0°C, 70wt% ethylamine aqueous solution (191.2g, 3mol) and 40wt% NaOH (300g, 3mol) aqueous solution were mixed, followed by dropwise addition of 2-isopropyl-5-methylbenzoyl chloride (300g, 1.5mol) Methyl tert-butyl ether solution (3000mL). After the reaction, wash with water (300mL*3), distill under reduced pressure, collect the 95-98℃ / 150pa fraction to obtain N-ethyl-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com