Preparation method of 3-alkyl indolizine derivative
A technology for indene derivatives and derivatives is applied in the field of organic synthetic chemistry, and can solve the problems of many steps, direct purchase, difficulty, etc., and achieve the effects of simple process flow, cheap and easy availability, and reduced production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
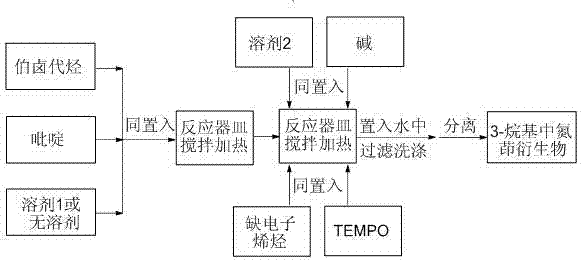
[0041] as attached figure 1 According to the technological process, 48 microliters of pyridine (equivalent to 0.60 mmol) and 65 microliters of 1-bromobutane (equivalent to 0.60 mmol) were put into the reaction vessel, and 0.20 ml of N , N - Dimethylformamide, heated and stirred at 80 degrees for 2.0 hours, then added 19.2 mg of lithium hydroxide (equivalent to 0.80 mmol), 125.0 mg of TEMPO (equivalent to 0.80 mmol), N , N - Put 21 microliters of dimethylacrylamide (equivalent to 0.20 mmol) and 2.8 milliliters of dimethyl sulfoxide into a reaction vessel, heat and stir at 140 degrees Celsius for 24 hours until the reaction is complete, pour the reacted mixture into water, and filter , washed and isolated to obtain 32.7 mg of the target product of this example (71% yield).
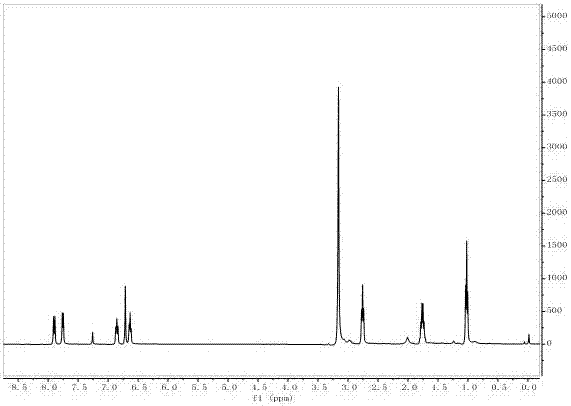
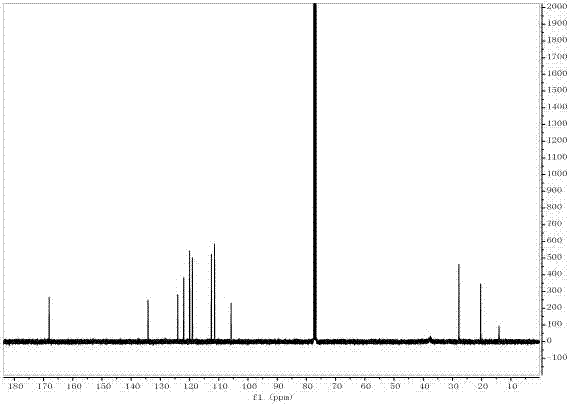
[0042] The target product in Example 1 was analyzed by a nuclear magnetic resonance spectrometer (model: AVANCE 400MHz, manufacturer: Bruker, Switzerland) to obtain figure 2 The H NMR spectra shown an...
Embodiment 2
[0044] as attached figure 1 According to the technological process, 93.1 mg (equivalent to 0.60 mmol) of 4-phenylpyridine and 91.8 mg (equivalent to 0.60 mmol) of 1-bromo-3-methoxypropane are put into the reaction vessel together, without Add solvent 1, heat and stir at 90°C for 1.0 hour, then add 180 mg of cesium hydroxide (equivalent to 1.20 mmol), 156 mg of TEMPO (equivalent to 1.0 mmol), and 13 microliters of acrylonitrile (equivalent to 0.20 mmol) and 2.0 mL N , N - Put dimethylformamide into a reaction vessel, heat and stir at 150 degrees Celsius for 24 hours until the reaction is complete, pour the reacted mixture into water, filter, wash, and isolate 47.5 mg of the target product of this example (yield: 86 %).
Embodiment 3
[0046] as attached figure 1 According to the technological process, take 3,5-lutidine as 64.3 mg (equivalent to 0.60 mmol), 2-bromomethyl-1,3-dioxolane as 100 mg (equivalent to 0.60 mmol), and Put it into a reaction vessel, add 0.2 ml of 1,4-dioxane, heat and stir at 100 degrees for 3.0 hours, then add 80 mg of lithium tert-butoxide (equivalent to 1.00 mmol), 313 mg of TEMPO (equivalent to 2.0 mmol), dimethyl maleate 43.2 mg (equivalent to 0.30 mmol) and 3.0 ml N -Put methylpyrrolidone into a reaction vessel, heat and stir at 130 degrees Celsius for 36 hours until the reaction is complete, pour the reacted mixture into water, filter, wash, and isolate 72.0 mg of the target product of this example (yield: 72%) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com