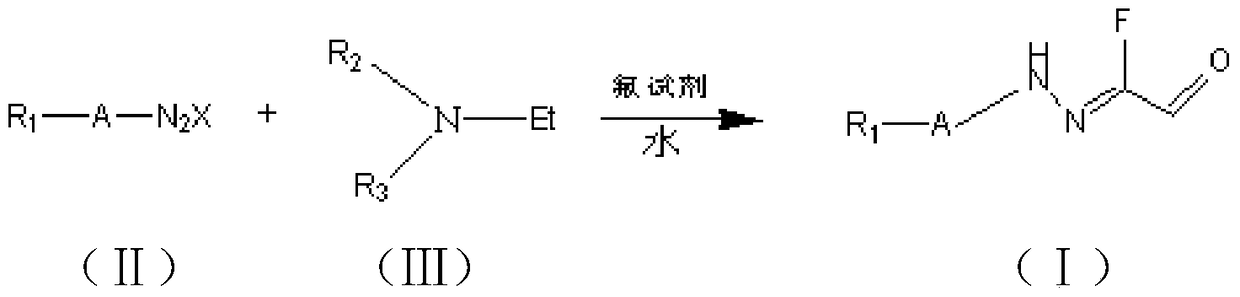
Fluorine-containing compound and its synthesis method
A synthetic method and compound technology, applied in organic chemistry, drug combination, hydrazone preparation, etc., can solve problems affecting drug safety, cumbersome operation, difficult to guarantee, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
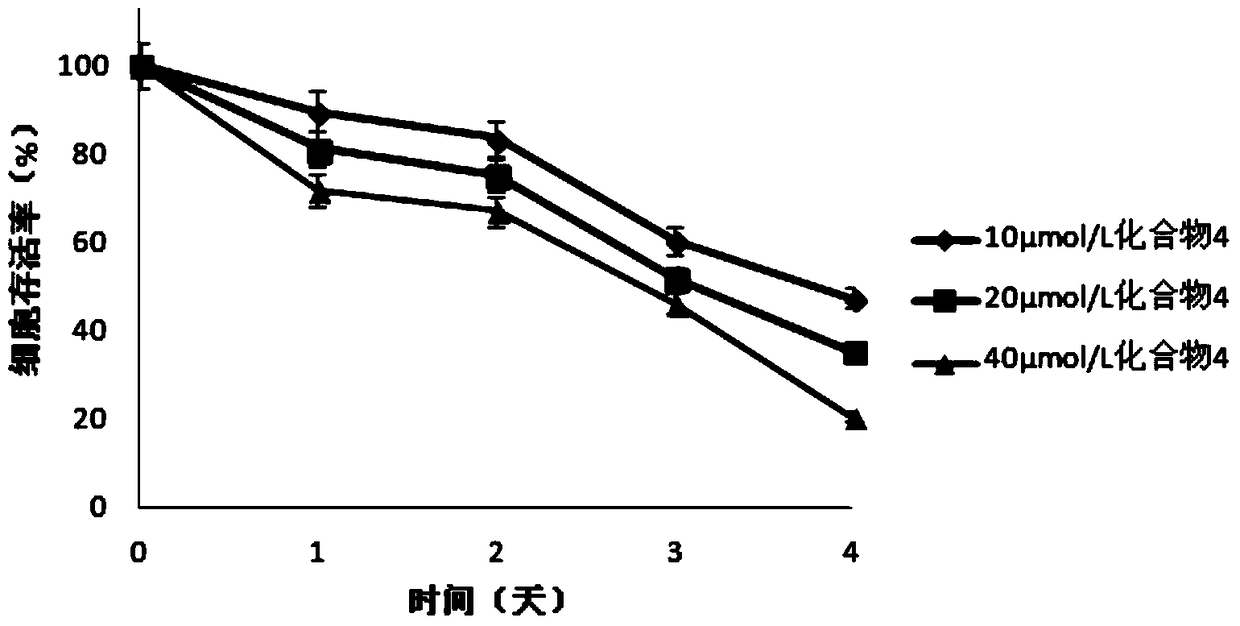
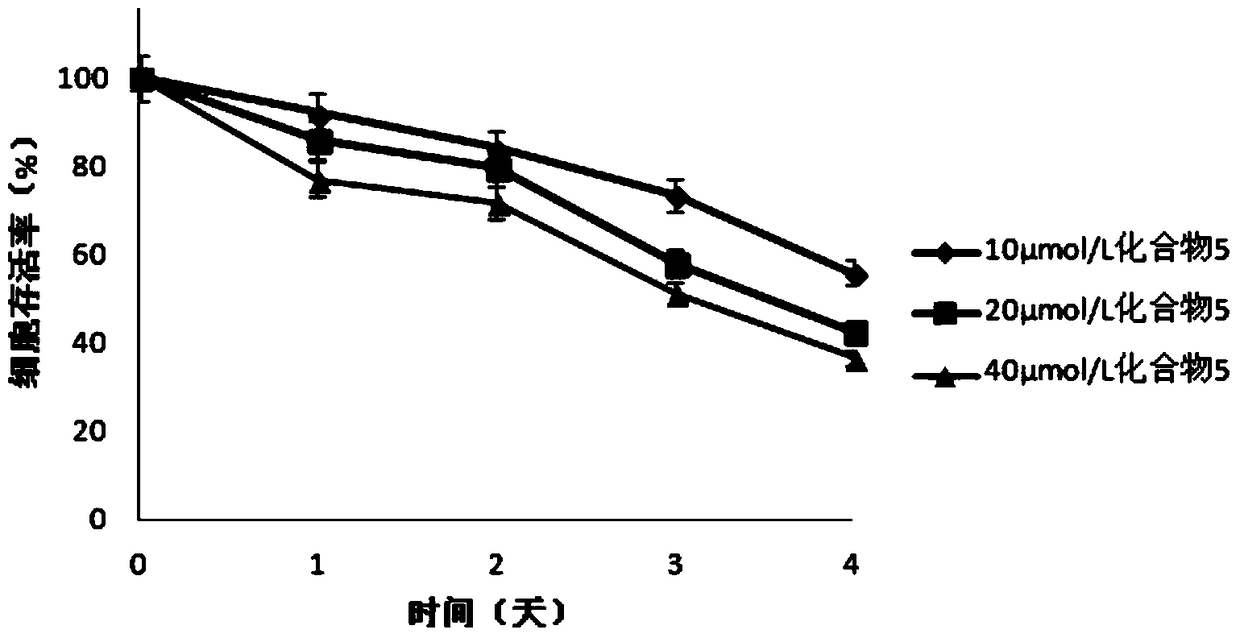
Image
Examples
Embodiment 1
[0112] 1-fluoroglyoxal-l-(4-fluorophenyl)hydrazone (2a)
[0113]
[0114] At room temperature, 4-fluorobenzenetetrafluoroborate diazonium salt (1a, CAS number: 459-45-0) (126mg, 0.600mmol, 1.00equiv), water (100μL, 0.560mmol, 0.930equiv) and Selectfluoro ( 952mg, 2.70mmol, 4.50equiv) was added to a mixed solvent (4.00mL) of DMA and toluene (v / v=3:1), and then N,N-diethyl-2-trifluoromethyl- Benzylamine (480 μL, 2.40 mmol, 4.00 equiv). After the dropwise addition, the stirring reaction was continued for 10 minutes, the system was washed with water (60.0 mL), and extracted twice with ethyl acetate (20.0 mL×2). The organic phases were combined and washed with anhydrous MgSO 4 Dry, filter, concentrate under vacuum, and separate by column chromatography (eluent ratio: n-hexane / ethyl acetate=19 / 1) to obtain 72.0 mg of the target compound (yield: 65%).
[0115] R f =0.40 (n-hexane / ethyl acetate 15:1 (v / v)).NMR Spectroscopy: 1 H NMR (400MHz, CDCl 3 )δ9.18(d, J=22.2Hz, 1H), 8.46...
Embodiment 2
[0117] 1-fluoroglyoxal-l-(4-bromophenyl)hydrazone (2b)
[0118]
[0119]At room temperature, 4-bromobenzenetetrafluoroborate diazonium salt (CAS number: 673-40-5) (162mg, 0.600mmol, 1.00equiv), water (100μL, 0.560mmol, 0.930equiv) and Selectfluoro (952mg, 2.70mmol, 4.50equiv) was added to a mixed solvent of DMA and toluene (v / v=3:1) (4.00mL), and then N,N-diethyl-2-trifluoromethyl-benzene was slowly added dropwise Amine (480 μL, 2.40 mmol, 4.00 equiv). After the dropwise addition, the stirring reaction was continued for 10 minutes, the system was washed with water (60.0 mL), and extracted twice with ethyl acetate (20.0 mL×2). The organic phases were combined and washed with anhydrous MgSO 4 Dry, filter, concentrate in vacuo, and separate by column chromatography (eluent ratio: n-hexane / ethyl acetate=19 / 1) to obtain 89.0 mg of the target compound (yield: 61%).
[0120] Rf=0.30 (n-hexane / ethyl acetate 15:1 (v / v)).NMR Spectroscopy: 1H NMR (400MHz, CDCl3) δ9.21 (d, J=22.3Hz,...
Embodiment 3
[0122] 1-fluoroglyoxal-l-(4-chlorophenyl)hydrazone (2c)
[0123]
[0124] At room temperature, 4-chlorobenzenetetrafluoroborate diazonium salt (CAS number: 673-41-6) (136mg, 0.600mmol, 1.00equiv), water (100μL, 0.560mmol, 0.930equiv) and Selectfluoro (952mg, 2.70mmol, 4.50equiv) was added to a mixed solvent of DMA and toluene (v / v=3:1) (4.00mL), and then diisopropylethylamine (CAS No.: 7087-68-5) was slowly added dropwise ( 397 μL, 2.40 mmol, 4.00 equiv). After the dropwise addition, the stirring reaction was continued for 10 minutes, the system was washed with water (60.0 mL), and extracted twice with ethyl acetate (20.0 mL×2). The organic phases were combined and washed with anhydrous MgSO 4 Dry, filter, concentrate under vacuum, and separate by column chromatography (eluent ratio: n-hexane / ethyl acetate=19 / 1) to obtain 71.3 mg of the target compound (yield: 60%).
[0125] R f =0.40 (n-hexane / ethyl acetate 15:1 (v / v)).NMR Spectroscopy: 1 H NMR (400MHz, CDCl 3 )δ9.20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com