Composite photocatalytic system CQDS-KNbO3, preparation method and application thereof
A photocatalytic and composite technology, applied in the field of photocatalysis, can solve the problems of large band gap, high probability of recombination of holes and photogenerated electrons, inability to effectively use the visible part of sunlight, etc., and achieve strong redox Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1 Composite photocatalytic system CQD S -KNbO 3
[0014] 1. KNbO 3 Preparation of granules:
[0015] Preparation of KNbO by Hydrothermal Method 3 particles. 3.57g Nb 2 o 5 The powder and 37.69 g of KOH flake solid were added to a beaker with 19 ml of deionized water and stirred on a magnetic stirrer for 30 min until Nb 2 o 5 Mix well with KOH and dissolve all. Then the mixture was sealed in a polytetrafluoroethylene-lined stainless steel autoclave and heated at 160 °C for 12 h. Cool to room temperature. The product was ground into granular form, put into a muffle furnace for calcination, the calcination temperature was 400° C., and the calcination time was 1 h. Cool to room temperature and grind to get KNbO 3 particles.
[0016] 2. Preparation of carbon quantum dots CQDs:
[0017] Preparation of CQDs by Hydrothermal Method S . Add 1.6 g of ascorbic acid, 15 ml of ethylene glycol and 25 ml of deionized water into the beaker and stir for 30 minutes...
Embodiment 2
[0020] Example 2 Composite photocatalytic system CQD S -KNbO 3 Catalytic degradation of crystal violet under visible light
[0021] (1) Effect of light time on photocatalytic degradation of crystal violet
[0022] Visible light catalytic degradation: take 50.0mL 10.0mg / L crystal violet solution in a 100mL Erlenmeyer flask, add the CQD prepared in Example 1 S -KNbO 3 50.0mg, irradiated under visible light for 1.0~5.0h. Filter and measure its UV spectrum at 200-800nm. Take the absorbance at 582nm to calculate the degradation rate of crystal violet.
[0023] Degradation rate (%) = (C 0 –C) / C 0 ×100% (where C 0 : the concentration of the stock solution; C: the concentration of the sample). The results are shown in Table 1.
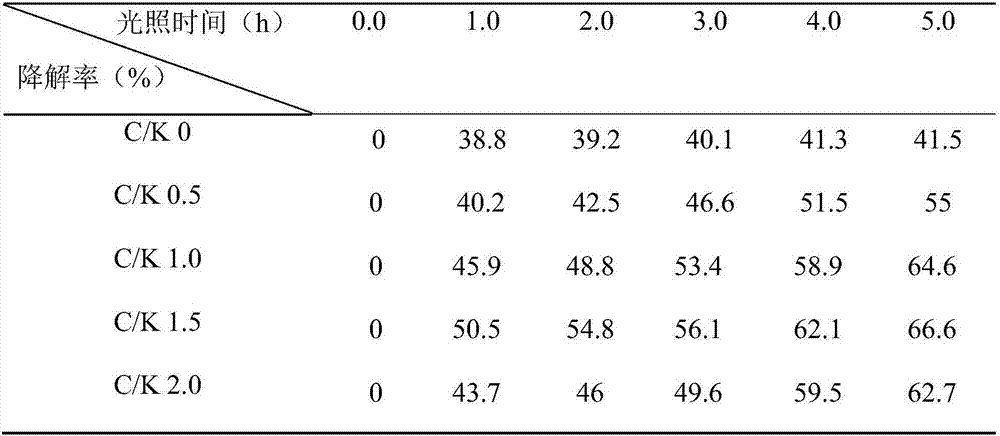
[0024] Table 1 Composite photocatalytic system CQD S -KNbO 3 Visible light photocatalytic degradation of crystal violet
[0025]
[0026] As shown in Table 1, with the prolongation of the illumination time, the degradation rates of catalysts C / K...
Embodiment 3
[0032] Example 3 Composite photocatalytic system CQD S -KNbO 3 Photocatalytic hydrogen production using crystal violet as a sacrificial agent
[0033] (1) Effect of light time on photocatalytic hydrogen production with crystal violet as sacrificial agent
[0034] Method: Measure 500mL of 50.0mg / L crystal violet solution into a photocatalytic hydrogen production reactor, add 200.0mg of the catalyst prepared in Example 1, and irradiate under visible light for 1.0-5.0h. Gas chromatography was used to measure the amount of hydrogen generated during the reaction. The results are shown in Table 3.
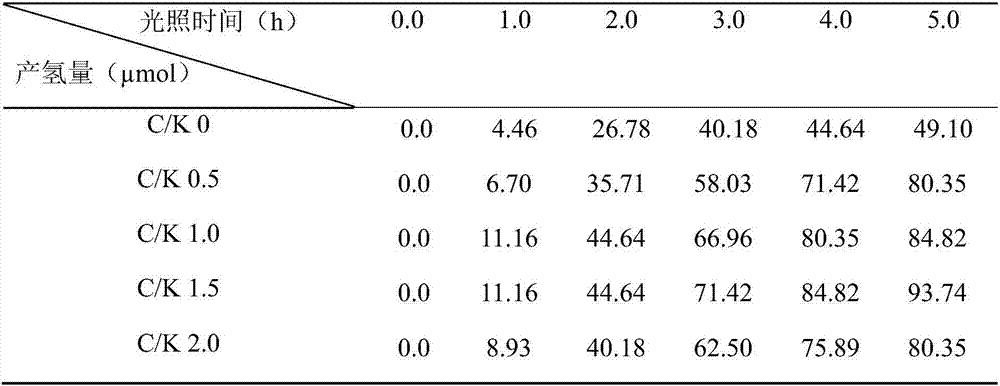
[0035] Table 3 Composite system CQD S -KNbO 3 Visible light photocatalytic hydrogen production
[0036]
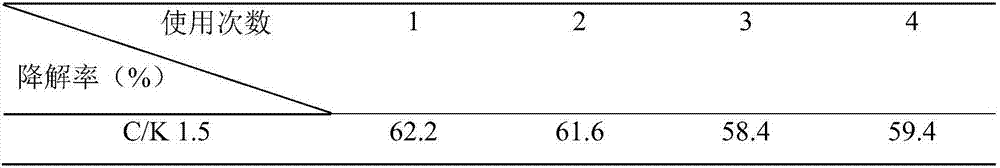
[0037] As shown in Table 3, the hydrogen production of catalysts C / K 0, C / K 0.5, C / K 1.0, and C / K 1.5 all increased with the increase of CQDs concentration with the prolongation of light time. When the C / K is 1.5 and the light time is 5.0h, the amount of hydrogen produced r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com